Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Selenium still represents a matter of debate in the scientific community. Bionanotechnology has introduced a whole new perspective on selenium use in animal nutrition.
- nanoparticles
- selenium
- animal nutrition
1. Introduction
An appropriate animal diet and living environment play a key role in animal health and performance. Thus, optimizing these factors is important for increasing rearing efficiency, which can positively determine the quality of production of animal origin. Over the last decade, nanotechnology has received the attention of many researchers due to its promising agricultural and food applications. Nanotechnology provides new “intelligent” solutions in animal nutrient delivery and health protection, and, indeed, it has the large potential to improve animal production systems [1]. This interest is mainly caused by the unique physicochemical properties of nanoparticles (NPs), which refers to their small size (1–100 nm), high stability, hydrophobicity, and large surface area. NPs’ hydrophobicity is important for good dispersion in water or serum and is also required to enhance their interaction with cell membranes [2]. The NPs’ size affects the cellular intake and allows them to easily pass through the stomach wall and diffuse into body cells quicker than common elements with larger particle sizes. The in vitro absorption of NPs with a diameter of 0.1 µm was found to be higher than 1 and 10 µm NPs [3]. The thickness of gastric mucus layers (total mucus), which continuously cover the gastrointestinal tract’s (GIT) surface, varies from 200 µm in the small intestine to 480–800 µm in the large intestine [4] and could allow the transport of NPs through the layer. According to Corbo et al. [5], NPs, especially nanominerals (e.g., Se and Zn), have a higher surface-area-to-volume ratio, providing more surface area for contact with the mucosal tissues and cells. Better absorption of NPs into the mucosal surface increases the particle residence time in the GIT. When a nanomineral is introduced into a biological medium, such as blood or mucus, proteins adsorb on its surface, giving it a unique “biological identity”, a so-called protein corona, which can have an impact on the NPs’ distribution as well as their potential toxicity [6]. Nanoparticles have been used in animal nutrition for their antibacterial, antifungal, and antioxidant properties as well as probiotics and to maintain general animal performance and health. The antimicrobial activities of metallic NPs (e.g., ZnO, CuO, and AgNPs) and SeNPs have been demonstrated by different researchers [7][8][9][10].
Selenium nanoparticles (SeNPs) are nano-sized (generally <60 nm in diameter) elemental selenium particles with excellent nano-properties [11]. For the NPs’ synthesis, there are two main strategies used: bottom-up (including chemical vapor deposition, hydrothermal and solvothermal methods, chemical reduction, and green synthesis) and top-down (including mechanical milling, laser ablation, etching, sputtering, and electro-explosion). The top-down strategy involves the mechanical breaking down of the bulk material into nanostructured materials. In contrast, the bottom-up method uses chemical reactions to break bulk into several parts to form NPs [12]. Methods of NPs synthesis can also be divided into physical, chemical, and biological (the so-called “green way” or “green synthesis”). The chemical methods of nanoparticle synthesis are the most common approaches commercially employed in various areas of NP applications. Concurrently, plenty of research indicates a potential environmental threat of nanotechnology related to NP toxicity [13][14][15][16][17]. The chemical approach to NP synthesis is related to the use of toxic chemicals, which are hazardous to humans and the environment [18]. Designing NPs via a green route using biological and eco-friendly materials reduces the negative environmental impact [19].
Various studies have investigated the possibilities of using selenium nanoparticles as a new source of selenium. For instance, sodium selenite NPs coated with methacrylate polymers were orally supplemented with ruminants, improving selenium absorption [20]. Shi et al. [21] stated that dietary nano-sized Se improved Se content in the blood and tissues and enhanced ruminal fermentation and feed utilization in sheep, which were fed a basal diet supplemented with 0.3, 3, and 6 g/kg DM of nano-Se. Kojouri et al. [22] reported the positive effect of dietary SeNPs inclusion (0.1 mg/kg DM for 60 days) on the antioxidant activity and weight gain of young lambs. In another experiment, the inclusion of 1 mg/kg DM of nano-sized Se into sheep’s diet exhibited a better antioxidative effect after 20–30 days of supplementation [23]. Xun et al. [24] also reported enhanced rumen fermentation and feed conversion efficiency in sheep supplemented with 4 mg/kg DM of nano-sized Se compared with selenium yeast (SY). In another experiment, supplementation with 0.5 mg/kg DM of nano-sized Se improved hair follicle development and promoted growth in Cashmere goats [25]. Experiments with nano-Se inclusion in broiler chicken diets conducted by Gangadoo et al. [26][27] demonstrated improved gut health and general animal performance; the best results in both experiments were obtained with an SeNP supplementation of 0.9 mg/kg DM with no toxic effect occurring. Previous studies have demonstrated the benefits of using SeNPs in broiler feed, with increased absorption and diffusion of material into organs and tissues, increased antioxidant capacity, and meat quality.
In contrast, Wang et al. [28] did not observe any beneficial effect of SeNP supplementation in terms of enhancing the oxidative status in broilers, but Se improved the survival rates. Gulyas et al. [29] reported changes in the proteome profile in chickens after SeNPs supplementation. These results could be related to the specific patented method of NP preparation used in this study. Several studies reported improvements in the growth performance [30][31][32], intestinal health [33][34], and antioxidant status [35] of aquatic animals supplemented with SeNPs. Se supplementation alleviated the antioxidant balance and enhanced kidneys cells’ resistance to oxidative damage in grass carp [36]. In another study, SeNP supplementation improved intestinal health, feed utilization, and growth performance in Nile tilapia [34]. The enhancement of the growth performance and feed efficiency after SeNP supplementation (0.4–0.8 mg) in Nile tilapia was also observed by Ibrahim et al. [37]. Markedly, the nanoform of Se can enhance growth performance in fish. The recommended dosage of SeNP dietary inclusion ranges from 0.15 to 4 mg/kg depending on the fish species [38].
Compared to selenite and selenate, SeNPs are more biocompatible and less toxic to animal organisms [4]. Nevertheless, high doses or long-term supplementation of SeNPs may lead to adverse effects in animal organisms and can be toxic. Several in vivo studies were conducted to measure NPs toxicity. Urbankova et al. reported that SeNPs supplementation had fewer negative effects in rats compared to the standard form. In contrast, supranutritional doses of SeNP administration (0.2, 0.4, and 0.8 mg/kg of body weight) showed a positive effect on reproductive functions and immune and antioxidant capacity. Other experiments on mice and rats supplemented with SeNPs demonstrated the hepatotoxic effect of SeNPs, which were also confirmed by further histological examination [39][20][40][41][42]. Damage to the liver parenchyma and intestinal epithelium in rats was reported after 0.5, 1.5, 3, and 5 mg/kg DM of SeNP supplementation [43]. The authors suggest that short-term SeNP supplementation can be safer and more beneficial in specific treatments. This unfavorable effect could be related to the tested animals’ metabolisms, biological characteristics, and the correlation between animal weight and the dosage of NPs administrated. The toxicity of NPs can largely vary among different species [44]. SeNP hyperaccumulation in Pangasius hypophthalmus liver, brain, and muscles was observed after SeNP supplementation (2.5–4 mg/L), which caused oxidative stress and toxicity in fish [45]. In another study, SeNP (100 μg Se/L) supplementation in Oryzias latipes enhanced oxidative stress caused by the hyperaccumulation of Se in the liver [46].
Based on the studies mentioned above, SeNP supplementation can have many health benefits (e.g., improved production performance, growth, feed efficiency, antioxidant status, and immune status) when present in animal diets compared to inorganic Se sources. Nevertheless, high doses of SeNPs can cause the hyperaccumulation of Se in tissues and oxidative stress or toxicity. Therefore, SeNPs should be included in animal diets in optimum doses to formulate nutritionally balanced feeds. The mechanism of nano-sized Se conversion remains unclear, and the gut microbiota is thought to play a key role in this process. The application of SeNPs showed promising results in improving the oxidative status of the cell induced by a reduction in glutathione (GSH), superoxide dismutase (SOD) levels [47], and GPx activities [48]. Whereas the great advantage of SeNP application compared to sodium selenite can be increased availability of the element [49], on the other hand, this advantage could be turned into a disadvantage through uncontrolled SeNP penetration across cellular membranes, which might be harmful to animal health. According to Surai et al. [50], the metabolism and assimilation of nano-sized Se could be disadvantageous in the animal diet when Se’s main mechanism of biological activity is mediated via selenoprotein synthesis. Moreover, the effect of dietary SeNPs on gut health and the formation of the accumulated nano-sized Se in animal tissues after supplementation is still unknown and needs further investigation. Furthermore, the topic of whether SeNPs supplementation may increase Se stores in the body remains unanswered.
2. Green Synthesis of SeNPs
Over the past decade, the biological method of producing NPs has become an emerging trend in nanotechnology and was developed as a sustainable way to overcome the disadvantages of chemical-based NP synthesis (e.g., high cost and toxic chemicals usage) [6]. Green synthesis provides a new possibility to synthesize NPs via an eco-friendly approach using simple unicellular or multicellular biological entities (e.g., bacteria, fungi, yeast, algae, and plant tissues) as natural reducing and stabilizing agents. The biologically synthesized nanostructures offer substantially different properties such as good adhesion, tribologically good properties, optical and electrical properties, and many promising applications. In NP synthesis, reducing and capping agents play an important role in impacting useful NP properties such as size, morphology, stabilizing, and protecting the NPs’ surface, preventing aggregation and uncontrolled growth [51]. Chemical components (i.e., polyethylene glycol, formaldehyde, polyethyleneimine, and polyacrylic acid) used as capping, reducing, stabilizing agents, or solvents in the procedures of chemical NP synthesis are hazardous and extremely toxic [52][53]. To be easily utilized in the living systems and not cause cellular toxicity, capping agents should be nontoxic, biodegradable, biocompatible, biosoluble, and well dispersed [54]. Green capping agents (e.g., amino acids and polysaccharides) are environmentally friendly. They may lead to designing NPs with unique morphologies and sizes, which can improve, for example, drug delivery via NPs, thereby enhancing NPs’ antifungal, antiviral, and antibacterial activity. Nutrients in the form of nanoparticles can be encapsulated in nanocapsules and carried via GIT into the bloodstream and then into body organs, where they enhance the bioavailability of delivered nutrients [55]. Biological synthesis was successfully used to produce different metal NPs, such as AuNPs, FeNPs, and AgNPs [53][56][57][58][59]. Green synthesis was also employed to produce SeNPs [60][61][62][63], and their antimicrobial, antifungal properties, and cytotoxicity were tested in various in vitro studies.
3. Antimicrobial Potential of SeNp Produced via Green Synthesis
Antimicrobial resistance (AMR) represents a major global problem, which significantly affects human and animal health. The wide use of antibiotics as growth promoters in animal farming has caused the development of increased antibiotic resistance in numerous bacterial strains. As a consequence, in 2003, the use of antibiotics in livestock diets was banned in the EU [64]. AMR adversely affects animal health, leading to the poor quality of products of animal origin and economic losses. Therefore, finding a new solution to overcome antibiotic usage is strongly needed. In recent years, nanotechnology enabled the manufacture of effective antimicrobial agents from nano-scaled materials, particularly metals. Many studies confirmed the antioxidant, antibacterial, anticancer, and antifungal activities of metallic NPs [65][66][67][68]. Whereas selenium nanoparticles have attracted scientific interest primarily as a result of research into their anticancer properties; this nanomaterial’s antibacterial potential has recently been identified. NPs have a large surface area, which increases the area of interaction with pathogenic microorganisms. Furthermore, due to the nano size, they are more likely to enter bacterial surfaces.
Although most of the studies on the antimicrobial potential of biogenic SeNPs were conducted in vitro, the results of these studies showed noticeable antibacterial, antifungal, and anticancer SeNPs activities against many important humans and animal pathogens and their potential for future applications in nanomedicine and veterinary. Furthermore, SeNPs produced through the green way show lower cytotoxicity, greater bioavailability, and reactivity than inorganic and organic Se, which makes them an attractive candidate for future therapeutic applications. The therapeutic effect of biogenic SeNPs (2.5, 5.0, and 10.0 mg) was also confirmed in an in vitro experiment in a mouse model infected with Toxoplasma gondii with no cytotoxicity observed [9]. Based on in vitro studies, SeNPs represent a viable approach to inhibit bacterial growth without using antibiotics and to overcome the drawbacks of synthetic methods that employ toxic chemicals. Interesting results were obtained by Cremonini et al. [69] who demonstrated the significantly better antibacterial activity of biogenic SeNPs in comparison with chemically produced NPs.
The strengths, weaknesses, opportunities, and threats (SWOT) analysis for a brief overview of advances and weaknesses of SeNP application in animal nutrition is proposed in Figure 1.
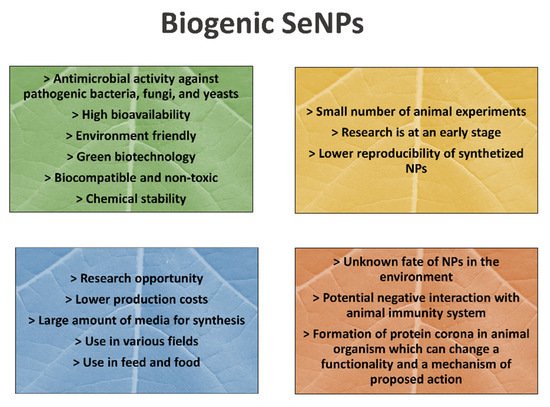
Figure 1. The strengths, weaknesses, opportunities, and threats (SWOT) analysis of SeNP application in animal nutrition.
4. Synthesis by Plants and Microorganisms
Live plants; plant tissues; and extracts from the plant leaf, latex, root, seed, and stem, or the whole plant have also been used to synthesize NPs, as they act as stabilizing or reducing agents [70][71]. Due to their genetic variability, plants possess many interesting metabolites, such as phenolic compounds, alkaloids, and sterols, that can serve as excellent biocapping and/or reducing agents. In NPs biosynthesis, plant polyphenols, which possess hydroxyl reducing groups, are usually used as stabilizing and reducing agents. Hydroxyl groups of biologically active plant compounds can also act as a capping agent by depositing on the NPs’ surfaces. Polyphenols and proteins may play a key role in reducing selenium ions to their element and stabilizing the SeNPs’ form [72]. Polysaccharides may effectively improve the NPs’ stability and morphology [73]. The different preparation methods of the plant extract from the same plant tissue may also significantly affect the shape, size, and distribution of NPs [74].
Singh et al. [74] used different Zingiber officinale rhizome extract preparation methods and obtained NPs with different properties. The significant advantage of plant-mediated NP synthesis is the inexpensiveness of culture compared to synthesis using microorganisms. In addition, it reduces the cost of microorganism isolation and further NP purification [75]. Moreover, plant-mediated NPs are stable, reproducible, environmentally friendly, and less time-consuming to produce [76]. Anu et al. [60] used Allium sativum extract to produce SeNPs and synthesized NPs 40–100 nm in size, showing decreased cytotoxicity compared to chemically produced SeNPs. SeNPs mediated from various plant extracts (e.g., Diospyros Montana, Murraya koenigii, Ephedra aphylla, and Thymus vulgaris) were reported to have antifungal, anticancer, and antimicrobial activity [10][61][77][78]. Green synthesis of SeNPs is commonly achieved by reducing selenate/selenite in the presence of bacterial proteins and plant extracts containing various metabolites such as phenols, flavonoids, alcohols, and proteins. Many microorganisms (e.g., Herbaspirillum sp., Bacillus arseniciselenatis, B. selenitireducens, and Comamonas testosteroni) have been observed to reduce toxic selenate and selenite into the nontoxic element selenium through aerobic or anaerobic conditions [70][79][80]. Microbes can produce NPs either intra- or extracellularly via different bioreduction processes using various microbial enzymes [71]. Microbial NP synthesis includes two reduction processes (reduction from selenate to selenium trioxide and then to elemental selenium), catalyzed by selenite and selenate reductases [81].
The study conducted by El-Saadony et al. [82] showed that SeNPs synthesized using Lactobacillus paracasei had an antagonistic effect against pathogenic fungi and significantly inhibited the growth of Candida and Fusarium species, which are the most known animal pathogenic species. Moreover, the diameter of obtained SeNPs ranges from 30 to 50 nm. In comparison, a previous study by Sasidharan and Balakrishnaraja [83] synthesized SeNPs by bacteria species (Lactobacillus casei; Streptococcus thermophilus; Bifidobacterium; Lactobacillus acidophilus; Lactobacillus helveticus; Klebsiella pneumoniae), but the disadvantage was the size of the produced NPs ranged from 50 to 550 nm. SeNPs synthesized using various cyanobacteria extracts (e.g., Nostoc sphericum, N. punctiforne, Spirulina pratensis, and Athrospira indica) showed good antioxidant activities and are recommended for future use as food supplements [84].
SeNPs can play an important role in eliminating microbial infections and, thus, improving animals’ growth and performance. SeNPs can inhibit both Gram-negative and Gram-positive bacteria by interrupting microbial biofilm [85] and possess significant antifungal activity by inhibiting spore germination [44]. The antifungal activity of SeNPs was tested mostly by in vitro experiments, and more extensive research in this field is needed. Shakibaie et al. [8] demonstrated a good potential of using bacteria Bacillus sp. for SeNPs synthesis. SeNPs prepared using these bacteria were orally administered to male mice, and biogenic SeNPs showed significantly less toxicity than synthetic SeNPs and SeO2.
Nevertheless, the reason for such a difference is not clear. Some in vivo experiments with biogenic SeNP dietary inclusion showed an improved oxidative status in tested animals without toxic effects [29][44][45]. Shirsat et al. [86] demonstrated a protective effect against the oxidative and immune stress of biogenic SeNPs synthesized using the bacteria Pantoea agglomerans in broilers’ diets. Song et al. obtained promising results, which used yeast Kluyveromyces lactis GG799 for SeNPs production. SeNPs demonstrated no toxicity in mice. Moreover, dietary supplementation with 0.6 mg/kg Se effectively attenuated oxidative stress, intestinal inflammation, and intestinal barrier dysfunction. However, these experiments are only a few, and further investigation of the impact of biogenic NPs on animals’ performance and production is required.
5. Synthesis of SeNPs by Marine Algae and Microalgae
Marine algae generally contain a wide spectrum of biologically active compounds such as polysaccharides, proteins, PUFA, various pigments, and antioxidants. Considering this spectrum, it predestines them to diverse commercial applications [87]. Marine algae may represent a novel nanotechnological solution that could facilitate the application of new alga-mediated NPs in medicine and animal nutrition. Some algae (e.g., Chlorella vulgaris, Sargassum wightii, Spirogyra insignis, Chondrus crispus, and Tetraselmis kochinensis) were used for the synthesis of metallic NPs such as Ag and Au NPs [88][89][90][91]. SeNPs synthesized via Spirulina pratensis showed antibacterial activity against foodborne microorganisms (Staphylococcus aureus and Salmonella typhimurium), but antibacterial activity increased with NP size reduction [92]. Aqueous extract of algae Sargassum angustifolium was used for biosynthesis of SeNPs, which were finally examined on antibacterial activity. Algae-coated SeNPs showed better antibacterial activity against Vibrio harveyi compared to uncoated SeNPs [93]. Algal cell walls are mainly composed of polysaccharides, natural polymers containing monosaccharides linked with glycosidic bonds. In recent years, the application of diverse algal polysaccharides (e.g., alginate and laminarin) has been reported [94]. Developing drug delivery systems using seaweed polysaccharides has received special attention in the scientific community due to the important field of biomedical research. Algal polysaccharides were successfully used for coating NPs as a stabilizing agent. The hydrophilic surface of functional groups on polysaccharides (e.g., hydroxyl, sulfate, and carboxyl groups) allows them to easily interact with biological tissues. Therefore, algal polysaccharides can serve as an excellent template for NP synthesis in modern nanotechnology.
Colloidal stability is frequently an issue that requires significant consideration since high agglomeration levels have been recorded in some situations [95]. The use of algae in NP production is also limited due to the lack of understanding of the synthesis mechanism. Studies regarding the employment of marine algae for SeNP production are still ongoing. It is believed to have a wide potential in the synthesis of biogenic NPs with interesting new properties.
This entry is adapted from the peer-reviewed paper 10.3390/agriculture11121244
References
- Horky, P.; Ruttkay-Nedecky, B.; Nejdl, L.; Richtera, L.; Cernei, N.; Pohanka, M.; Kopel, P.; Skladanka, J.; Hloucalova, P.; Slama, P.; et al. Electrochemical Methods for Study of Influence of Selenium Nanoparticles on Antioxidant Status of Rats. Int. J. Electrochem. Sci. 2016, 11, 2799–2824.
- Fratoddi, I. Hydrophobic and Hydrophilic Au and Ag Nanoparticles. Breakthroughs and Perspectives. Nanomaterials 2017, 8, 11.
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573.
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90.
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine (Lond.) 2016, 11, 81–100.
- Abdelnour, S.; Alagawany, M.; Hashem, N.; Farag, M.; Alghamdi, E.; Hassan, F.-u.; Bilal, R.; Elnesr, S.; Dawood, M.; Nagadi, S.; et al. Nanominerals: Fabrication Methods, Benefits and Hazards, and Their Applications in Ruminants with Special Reference to Selenium and Zinc Nanoparticles. Animals 2021, 11, 1916.
- Trang, H.D.N. Antibacterial properties of selenium nanoparticles and their toxicity to Caco-2 cells. Food Control 2017, 77, 17–24.
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241.
- Keyhani, A.; Zia-ali, N.; Shakibaie, M.; Kareshk, A.; Shojaee, S.; Asadi-Shekaari, M.; Sepahvand, M.; Mahmoudvand, H. Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J. Med. Microbiol. 2019, 69.
- El-Zayat, M.M.; Eraqi, M.M.; Alrefai, H.; El-Khateeb, A.Y.; Ibrahim, M.A.; Aljohani, H.M.; Aljohani, M.M.; Elshaer, M.M. The Antimicrobial, Antioxidant, and Anticancer Activity of Greenly Synthesized Selenium and Zinc Composite Nanoparticles Using Ephedra aphylla Extract. Biomolecules 2021, 11, 470.
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in Research on the Toxicological Effects of Selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726.
- Baig, N.; Kammakakam, I.; Falath, W.S. Nanomaterials: A review of synthesis, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871.
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061.
- Ramanathan, A. Toxicity of nanoparticles_ challenges and opportunities. Appl. Microsc. 2019, 49, 2.
- Maharramov, A.M.; Hasanova, U.A.; Suleymanova, I.A.; Osmanova, G.E.; Hajiyeva, N.E. The engineered nanoparticles in food chain: Potential toxicity and effects. SN Appl. Sci. 2019, 1, 1362.
- Elhardallou, S.; Babiker, W.; Sulieman, A.M.; Gobouri, A. Effect of Diet Supplementation with Food Industry By-Products on Diabetic Rats. Food Nutr. Sci. 2015, 6, 875–882.
- Boudreau, M.D.; Imam, M.S.; Paredes, A.M.; Bryant, M.S.; Cunningham, C.K.; Felton, R.P.; Jones, M.Y.; Davis, K.J.; Olson, G.R. Differential Effects of Silver Nanoparticles and Silver Ions on Tissue Accumulation, Distribution, and Toxicity in the Sprague Dawley Rat Following Daily Oral Gavage Administration for 13 Weeks. Toxicol. Sci. 2016, 150, 131–160.
- Khan, F.H. Chemical hazards of nanoparticles to human and environment (a review). Orient. J. Chem. 2013, 29, 1399.
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642.
- Romero-Pérez, A.; García-García, E.; Zavaleta-Mancera, A.; Ramírez-Bribiesca, J.E.; Revilla-Vázquez, A.; Hernández-Calva, L.M.; López-Arellano, R.; Cruz-Monterrosa, R.G. Designing and evaluation of sodium selenite nanoparticles in vitro to improve selenium absorption in ruminants. Vet. Res. Commun. 2010, 34, 71–79.
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Qiang, L.; Wang, Q.; Shi, L. Effect of elemental nano-selenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Fuel Energy Abstr. 2011, 163, 136–142.
- Kojouri, G.; Arbabi, F.; Mohebbi, A. The effects of selenium nanoparticles (SeNPs) on oxidant and antioxidant activities and neonatal lamb weight gain pattern. Comp. Clin. Pathol. 2020, 29, 369–374.
- Sadeghian, S.; Kojouri, G.A.; Mohebbi, A. Nanoparticles of selenium as species with stronger physiological effects in sheep in comparison with sodium selenite. Biol. Trace Elem. Res. 2012, 146, 302–308.
- Xun, W.; Shi, L.; Yue, W.; Zhang, C.; Ren, Y.; Qiang, L. Effect of High-Dose Nano-selenium and Selenium–Yeast on Feed Digestibility, Rumen Fermentation, and Purine Derivatives in Sheep. Biol. Trace Elem. Res. 2012, 150, 130–136.
- Wu, X.; Yao, J.; Yang, Z.; Yue, W.; Ren, Y.; Zhang, C.; Liu, X.; Wang, H.; Zhao, X.; Yuan, S.; et al. Improved fetal hair follicle development by maternal supplement of selenium at nano size (Nano-Se). Livest. Sci. 2011, 142, 270–275.
- Gangadoo, S.; Dinev, I.; Willson, N.L.; Moore, R.J.; Chapman, J.; Stanley, D. Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ. Sci. Pollut. Res. Int. 2020, 27, 16159–16166.
- Gangadoo, S.; Dinev, I.; Chapman, J.; Hughes, R.J.; Van, T.T.H.; Moore, R.J.; Stanley, D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl. Microbiol. Biotechnol. 2018, 102, 1455–1466.
- Wang, Y. Differential effects of sodium selenite and nano-Se on growth performance, tissue se distribution, and glutathione peroxidase activity of avian broiler. Biol. Trace Elem. Res. 2009, 128, 184–190.
- Gulyas, G.; Csosz, E.; Prokisch, J.; Javor, A.; Mezes, M.; Erdelyi, M.; Balogh, K.; Janaky, T.; Szabo, Z.; Simon, A.; et al. Effect of nano-sized, elemental selenium supplement on the proteome of chicken liver. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 101, 502–510.
- Kohshahi, A.J.; Sourinejad, I.; Sarkheil, M.; Johari, S.A. Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): Influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2019, 45, 793–804.
- Jahanbakhshi, A.; Pourmozaffar, S.; Adeshina, I.; Mahmoudi, R.; Erfanifar, E.; Ajdari, A. Selenium nanoparticle and selenomethionine as feed additives: Effects on growth performance, hepatic enzymes’ activity, mucosal immune parameters, liver histology, and appetite-related gene transcript in goldfish (Carassius auratus). Fish Physiol. Biochem. 2021, 47, 639–652.
- Liu, G.; Yu, H.; Wang, C.; Li, P.; Liu, S.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Nano-selenium supplements in high-fat diets relieve hepatopancreas injury and improve survival of grass carp Ctenopharyngodon Idella by reducing lipid deposition. Aquaculture 2021, 538, 736580.
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663.
- Ghazi, S.; Diab, A.M.; Khalafalla, M.M.; Mohamed, R.A. Synergistic Effects of Selenium and Zinc Oxide Nanoparticles on Growth Performance, Hemato-biochemical Profile, Immune and Oxidative Stress Responses, and Intestinal Morphometry of Nile Tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2021, 200, 364–374.
- Longbaf Dezfouli, M.; Ghaedtaheri, A.; Keyvanshokooh, S.; Salati, A.P.; Mousavi, S.M.; Pasha-Zanoosi, H. Combined or individual effects of dietary magnesium and selenium nanoparticles on growth performance, immunity, blood biochemistry and antioxidant status of Asian seabass (Lates calcarifer) reared in freshwater. Aquac. Nutr. 2019, 25, 1422–1430.
- Zhang, T.; Yao, C.; Hu, Z.; Li, D.; Tang, R. Protective Effect of Selenium on the Oxidative Damage of Kidney Cells Induced by Sodium Nitrite in Grass Carp (Ctenopharyngodon idellus). Biol. Trace Elem. Res. 2021.
- Ibrahim, M.S.; El-gendy, G.M.; Ahmed, A.I.; Elharoun, E.R.; Hassaan, M.S. Nanoselenium versus bulk selenium as a dietary supplement: Effects on growth, feed efficiency, intestinal histology, haemato-biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fingerlings. Aquac. Res. 2021, 52, 5642–5655.
- Dawood, M.A.O.; Basuini, M.F.E.; Yilmaz, S.; Abdel-Latif, H.M.R.; Kari, Z.A.; Abdul Razab, M.K.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium Nanoparticles as a Natural Antioxidant and Metabolic Regulator in Aquaculture: A Review. Antioxidants 2021, 10, 1364.
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268.
- Benko, I.; Nagy, G.; Tanczos, B.; Ungvari, E.; Sztrik, A.; Eszenyi, P.; Prokisch, J.; Banfalvi, G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ. Toxicol. Chem. 2012, 31, 2812–2820.
- He, Y.; Chen, S.; Liu, Z.; Cheng, C.; Li, H.; Wang, M. Toxicity of selenium nanoparticles in male Sprague-Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014, 115, 44–51.
- Hadrup, N.; Loeschner, K.; Mandrup, K.; Ravn-Haren, G.; Frandsen, H.L.; Larsen, E.H.; Lam, H.R.; Mortensen, A. Subacute oral toxicity investigation of selenium nanoparticles and selenite in rats. Drug Chem. Toxicol. 2019, 42, 76–83.
- Urbankova, L.; Skalickova, S.; Pribilova, M.; Ridoskova, A.; Pelcova, P.; Skladanka, J.; Horky, P. Effects of Sub-Lethal Doses of Selenium Nanoparticles on the Health Status of Rats. Toxics 2021, 9, 28.
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566.
- Kumar, N.; Krishnani, K.K.; Singh, N.P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. 2018, 25, 8914–8927.
- Li, H.; Zhang, J.; Wang, T.; Luo, W.; Zhou, Q.; Jiang, G. Elemental selenium particles at nano-size (Nano-Se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: A comparison with sodium selenite. Aquat. Toxicol. 2008, 89, 251–256.
- Hassanin, K.M.; Abd El-Kawi, S.H.; Hashem, K.S. The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int. J. Nanomed. 2013, 8, 1713–1720.
- Zhou, X.; Wang, Y.; Gu, Q.; Li, W. Effect of different dietary selenium source (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 2009, 291, 78–81.
- Yaghmaie, P.; Ramin, A.; Asri-Rezaei, S.; Zamani, A. Evaluation of glutathion peroxidase activity, trace minerals and weight gain following administration of selenium compounds in lambs. Vet. Res. Forum 2017, 8, 133–137.
- Surai, P.F.; Kochish, I.I. Food for thought: Nano-selenium in poultry nutrition and health. Anim. Health Res. Rev. 2020, 21, 103–107.
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600.
- Duan, H.; Wang, D.; Li, Y. Green Chemistry for Nanoparticle Synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792.
- Al-khattaf, F.S. Gold and silver nanoparticles: Green synthesis, microbes, mechanism, factors, plant disease management and environmental risks. Saudi J. Biol. Sci. 2021, 28, 3624–3631.
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.u.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406.
- Bhattarai, B.; Zaker, Y.; Bigioni, T.P. Green synthesis of gold and silver nanoparticles: Challenges and opportunities. Curr. Opin. Green Sustain. Chem. 2018, 12, 91–100.
- Cardoso-Avila, P.E.; Patakfalvi, R.; Rodríguez-Pedroza, C.; Aparicio-Fernández, X.; Loza-Cornejo, S.; Villa-Cruz, V.; Martínez-Cano, E. One-pot green synthesis of gold and silver nanoparticles using Rosa canina L. extract. RSC Adv. 2021, 11, 14624–14631.
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233.
- Muthusamy, A.; Pottail, L. Rapid Green Synthesis of Gold and Silver Nanoparticles Using Ethanol Extract of Kedrostis Foetidissima (JACQ) COGN. And Its Anticancer Efficacy against A549 Human Lung Cancer Cell Lines. Indian Drugs 2021, 58, 30–40. Available online: http://www.indiandrugsonline.org/issuesarticle-details?id=MTE1OQ (accessed on 3 November 2021).
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-Synthesis of Selenium Nanoparticles Using Garlic Cloves (Allium sativum): Biophysical Characterization and Cytotoxicity on Vero Cells. J. Clust. Sci. 2017, 28, 551–563.
- Kokila, K.; Elavarasan, N.; Sujatha, V. Diospyros montana leaf extract-mediated synthesis of selenium nanoparticles and their biological applications. New J. Chem. 2017, 41, 7481–7490.
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Muehlmann, L.A.; Deng, S.-e.; Wang, X.; Li, W.; Zhang, W. Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1463–1470.
- Salem, S.S.; Fouda, M.M.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J. Clust. Sci. 2021, 32, 351–361.
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1831 (accessed on 3 November 2021).
- Hassan, F.A.; Abdel-Azeem, N.; Abdel-Rahman, S.; Amin, H.F.; Abdel-Mawla, L.F. Effect of Dietary Organic Selenium Supplementation yfon Growth Performance, Carcass Characteristics and Antioxidative Status of Growing Rabbits. World Vet. J. 2019, 9, 16–25. Available online: www.wvj.science-line.com (accessed on 3 November 2021).
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870.
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour.-Effic. Technol. 2017, 3, 406–413.
- Horky, P.; Skalickova, S.; Urbankova, L.; Baholet, D.; Kociova, S.; Bytesnikova, Z.; Kabourkova, E.; Lackova, Z.; Cernei, N.; Gagic, M. Zinc phosphate-based nanoparticles as a novel antibacterial agent: In vivo study on rats after dietary exposure. J. Anim. Sci. Biotechnol. 2019, 10, 1–12.
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771.
- Zheng, S.; Su, J.; Wang, L.; Yao, R.; Wang, D.; Deng, Y.; Wang, R.; Wang, G.; Rensing, C. Selenite reduction by the obligate aerobic bacterium Comamonas testosteroni S44 isolated from a metal-contaminated soil. BMC Microbiol. 2014, 14, 204.
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100.
- Mellinas, C.; Jiménez, A.; Garrigós, M.D.C. Microwave-Assisted Green Synthesis and Antioxidant Activity of Selenium Nanoparticles Using Theobroma cacao L. Bean Shell Extract. Molecules 2019, 24, 4048.
- Fardsadegh, B.; Jafarizadeh, H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process. Synth. 2019, 8, 399–407.
- Singh, R.; Sadasivam, M.; Rakkiyappan, C. Ginger (Zingiber officinale) root extract: A source of silver NANOPARTICLES and their application. Int. J. Bio-Eng. Sci. Technol. 2021, 2, 75–80.
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2021.
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593.
- Yazhiniprabha, M.; Vaseeharan, B. In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mater. Sci. Eng. C 2019, 103, 109763.
- Pandiyan, I.; Sakthi, S.D.; Indiran, M.A.; Rathinavelu, P.K.; Rajeshkumar, S. Mediated Selenium Nanoparticles, Characterization and its Antimicrobial Activity-An In Vitro Study. Thymus Vulgaris 2021, 7, 3516–3521.
- Eswayah, A.S.; Smith, T.J.; Gardiner, P.H. Microbial Transformations of Selenium Species of Relevance to Bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859.
- Xu, X.; Cheng, W.; Liu, X.; You, H.; Wu, G.; Ding, K.; Tu, X.; Yang, L.; Wang, Y.; Li, Y.; et al. Selenate Reduction and Selenium Enrichment of Tea by the Endophytic Herbaspirillum sp. Strain WT00C. Curr. Microbiol. 2020, 77, 588–601.
- Afzal, B.; Fatma, T. Selenium Nanoparticles: Green Synthesis and Exploitation. In Merging Technologies for Nanoparticle Manufacturing; Patel, J.K., Pathak, Y.V., Eds.; Springer: Cham, Switzerland, 2021.
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.M.; Fouda, S.E.E.; El-Tahan, A.M.; et al. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471.
- Sasidharan, S.; Balakrishnaraja, R. Comparison Studies on the Synthesis of Selenium Nanoparticles by Various Micro-organisms. Int. J. Pure Appl. Biosci. 2014, 2, 112–117.
- Afzal, B.; Yasin, D.; Husain, S.; Zaki, A.; Srivastava, P.; Kumar, R.; Fatma, T. Screening of cyanobacterial strains for the selenium nanoparticles synthesis and their anti-oxidant activity. Biocatal. Agric. Biotechnol. 2019, 21, 101307.
- Hariharan, H.; Al-Harbi, N.; Karuppiah, P.; Rajaram, S.K. Microbial synthesis of selinium nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515.
- Shirsat, S.; Kadam, A.; Mane, R.; Jadhav, V.; Zate, M.; Highly Cited Researcher, M.; Kim, K. Protective Role of Biogenic Selenium Nanoparticles in Immunological and Oxidative Stress Generated by Enrofloxacin in Broiler Chicken. Dalton Trans. 2016, 45.
- Hadrová, S.; Sedláková, K.; Krizova, L.; Malyugina, S. Alternative and Unconventional Feeds in Dairy Diets and Their Effect on Fatty Acid Profile and Health Properties of Milk Fat. Animals 2021, 11, 1817.
- Singaravelu, G.; Arockiamary, J.; Kumar, G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B Biointerfaces 2007, 57, 97–101.
- Luangpipat, T.; Beattie, I.; Chisti, Y.; Haverkamp, R. Gold nanoparticles produced in a microalga. J. Nanopart. Res. 2011, 13, 6439–6445.
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater. Lett. 2012, 79, 116–118.
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013, 7, 109–116.
- ElSaied, B.; Diab, A.; Tayel, A.; Alghuthaymi, M.; Moussa, S. Potent antibacterial action of phycosynthesized selenium nanoparticles using Spirulina platensis extract. Green Process. Synth. 2021, 10, 49–60.
- Mansouri-Tehrani, H.A.; Keyhanfar, M.; Behbahani, M.; Dini, G. Synthesis and characterization of algae-coated selenium nanoparticles as a novel antibacterial agent against Vibrio harveyi, a Penaeus vannamei pathogen. Aquaculture 2021, 534, 736260.
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Seaweed polysaccharide-based nanoparticles: Preparation and applications for drug delivery. Polymers 2016, 8, 30.
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498.
This entry is offline, you can click here to edit this entry!
