Although there is growing data on the acute and chronic health benefits of cow milk, albeit not yet conclusive, whether milk from alternative (non-bovine) sources could provide comparable or superior cardiometabolic protection has not yet been comprehensively reviewed. This narrative review outlines the marked differences in macronutrient composition, particularly protein and lipid content, and discusses how whole milk product (and individual milk ingredients) from different species could impact cardiometabolic health.
- milk
- cardiometabolic health
- metabolism
- glycaemia
- energy expenditure
- appetite
- obesity
- type II diabetes
1. Cow Milk Consumption and Cardiometabolic Health
The consumption of cow dairy products is a dominant feature in the diet of many cultures globally, particularly among Western communities. There is some evidence from epidemiological studies and systematic reviews alike that dairy intake is inversely linked with the risk of developing metabolic syndrome [1–3]. More pertinently, a body of data supports a negative association between milk intake and the risk of developing dysglycaemia, dyslipidaemia, and hypertension [1,4]. However, with gold-standard data from long-term randomised controlled trials (RCTs) featuring type II diabetes (T2D) and cardiovascular disease (CVD) incidence as primary endpoints not currently available, the causality of these findings remains to be confirmed [5]. Nonetheless, putative explanations for a possible metabolic syndrome risk reduction include a direct modulation of the glycaemic response [2,6], and an indirect modulation of body weight through upregulation of postprandial thermogenesis [6–8] and/or suppression of appetite [9–11]. Features of, or responses to, milk that might contribute to any cardiometabolic protection include the bioactive peptide content [12,13]; fatty acid (FA) content [14], e.g., conjugated linoleic acid (CLA) [15]; glycaemic index (GI) [16,17]; promotion of satiety [18]; mineral content, particularly calcium, magnesium, and potassium [19–22]; and folate bioavailability [23].
Although there is growing data on the acute and chronic health benefits of cow milk, albeit not yet conclusive, whether milk from alternative (non-bovine) sources could provide comparable or superior cardiometabolic protection has not yet been comprehensively reviewed.
2. Cow Milk Alternatives
The worldwide commercial production of cow milk decisively eclipses the relatively minor contributions from alternative animal species (Table 1). Nonetheless, these milks remain valuable primary sources for many countries and communities globally.
Table 1. Mean contribution of individual species’ milks towards global production [24].
|
Milk Origin |
Global Milk Production (%) |
Global Milk Production (kg) |
|
Cow |
81.3 |
714,400,000,000 |
|
Buffalo |
14.8 |
130,300,000,000 |
|
Goat |
2.2 |
18,900,000,000 |
|
Sheep |
1.3 |
11,800,000,000 |
|
Camel |
0.4 |
3,200,000,000 |
Values rounded to nearest 0.1 percent or 109 kg.
Owing to the specific make-up of proteins (e.g., β-lactoglobulin; β-lg) and sugars (e.g., lactose) within cow milk, the global prevalence of cow milk allergy and intolerance is notably high. Approximately 65% of adults worldwide have a suboptimal capacity to digest and absorb lactose [25]. In Asian and American Indian populations, the reported prevalence of lactose intolerance is closer to 100% [26,27]. However, with marked compositional differences, hypoallergenicity and improved tolerability have been indicated following the ingestion of goat [28], sheep [29], camel [30], buffalo [31], and donkey [32] milk, as compared to cow milk. It should be noted that throughout this review buffalo milk refers to the produce of animals of the Bubalus genus.
Lastly, non-dairy substitutes for milk, including soy, oat, rice, and nut ‘milk beverages’ have received growing attention. These plant-based alternatives are formulated through the disintegration of plant material, extraction in water, and subsequent homogenisation, which produces a ‘milk’ reminiscent of the consistency and appearance of animal milk [33]. Despite a typically substandard macronutrient profile relative to mammalian milk, plant-based ‘milks’ possess distinct functional ingredients, lower allergenicity and greater affordability, which have impelled a noticeable surge in demand and production.
3. The Composition and Digestibility of Milks of Different Origin
3.1. Composition
With knowledge of varying nutritional profiles, beyond any implications of a reduced allergenic potential of non-bovine milk, the ingestion of milk from different species could also engender distinct health benefits. Marked differences have been documented in macronutrient composition between multiple milk sources, particularly in terms of protein and lipid content. For instance, 100 g of sheep milk provides a markedly greater amount of protein (P: 5.5 g) and fat (F: 5.9 g) compared to cow (P: 3.4 g; F: 3.3 g), goat (P: 3.7 g; F: 3.8 g), and camel (P: 3.3 g; F: 4.0 g) milk [22,29]. Buffalo and reindeer milks also have a notably high lipid content (7.4 g/100 g and 16.1 g/100 g, respectively) [34]. In addition, mean lactose content varies modestly across ruminant milks at 4.51%, 4.75%, 4.79%, and 4.82% for 100 g of goat, sheep, buffalo, and cow milk, respectively [35]. See Figure 1 for a comparison of the differing macronutrient profiles of common animal milks, and Table 2 for a more detailed examination of the nutritional composition between different animal milks and plant-derived milk alternatives. It is noted that these cited values, and those in the following sections, should merely serve as typical examples of a milk’s nutritional composition. This caveat is raised with knowledge that milk composition can greatly vary under different conditions (i.e., protein composition is largely determined by genetics, and thus varies with herds; whilst lipid content is largely determined by environment, and thus varies with forage and season). Multiple studies with similar designs yet discordant findings have epitomised this variability of milk composition with animal breed, age, health status, diet, and lactation stage, or even milking yield/time of day [36,37]. For instance, the proportion of lipid content ascribed to either unsaturated or saturated FAs in mare (i.e., equine) milk can vary considerably across breed and lactation stage (unsaturated: 39–62%; saturated: 38–61%) [36]. Environmental pollutants may also alter the properties of milk fat [38]. Hence, great caution should be taken when drawing conclusions from a single study’s findings, especially when this is in the form of low-level evidence from analytical studies such as those cited above.
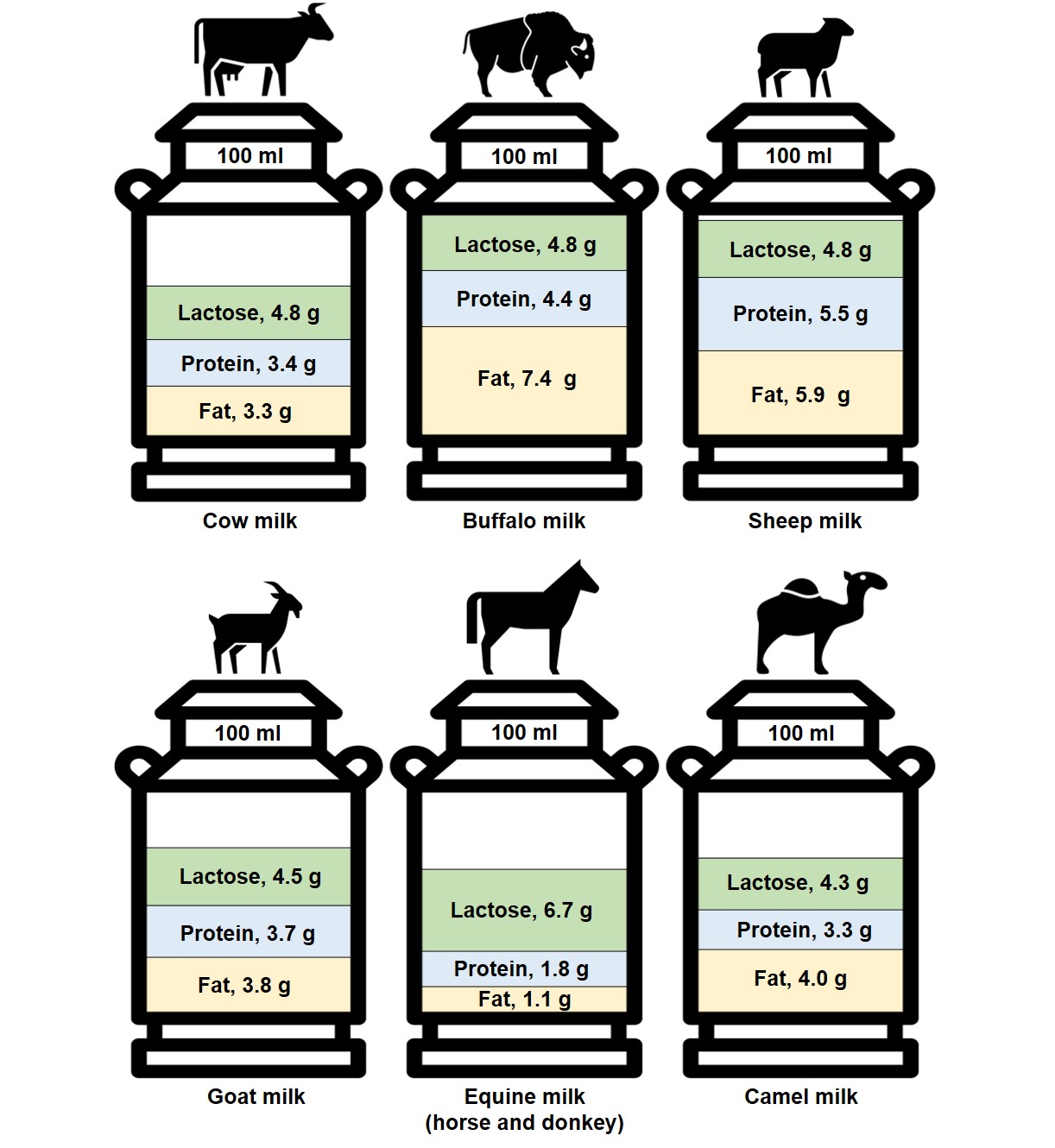
Figure 1. The composition of different species’ milk by fat, protein, and lactose content per 100 mL [22,29,34,35]. Equine milk values represent the mean nutrient content in mare and donkey milks.
Besides gross protein, fat, and carbohydrate quantity, macronutrient quality can also greatly differ between milks of different origin, further altering implications for human health. Relative to monogastric mammals such as humans and mares, casein forms a far greater portion of the protein content in ruminant milk [22]. Moreover, comparisons across ruminant milks have documented that the casein fraction of sheep, goat, buffalo, and yak milk largely comprises β-casein, whilst αS1-casein predominates in cow milk [22,39]. Alongside αS1-casein and β-casein, αS2-casein and κ-casein complete the group of different casein phosphoproteins in mammalian milk. These four phosphoproteins are defined by their distinct primary amino acid (AA) sequence, micellar position, and subsequently, function (e.g., calcium and phosphate transportation, stability and solubility) [40]. Although whey, a by-product of the cheese-making process, is also a family of (five) heterogeneous and polymorphic protein fractions, the composition of whey is more consistent across species, with a common preponderance of β-lg, except in camel milk—in which serum albumin dominates, and β-lg is largely absent [22].
Table 2. Nutritional composition per 100 mL milk of different animal-derived milk and plant-based milk alternatives.
|
|
Milk Origin |
|||||||||
|
Cow |
Buffalo |
Sheep |
Goat |
Equine |
Camel |
Soy |
Oat |
Rice |
Almond |
|
|
Total fat (%) |
3.3 |
7.4 |
5.9 |
3.8 |
1.1 |
4.0 |
2.0 |
2.2 |
1.0 |
1.1 |
|
MCT (% of |
10.5 |
7.1 |
21.8 |
23.0 |
15.2 |
1.5 |
n.d. |
n.d. |
n.d. |
0.2 |
|
CLA (% of |
0.7 |
0.5 |
1.2 |
0.6 |
0.1 |
0.9 |
n/a |
n/a |
n/a |
n/a |
|
SFA (% of |
68.4 |
70.8 |
65.0–75.0 |
65.0–73.8 |
38.0–61.0 |
66.1 |
14.3 |
18.9 |
12.0 |
22.6 |
|
MFG diameter (µm) |
3.8 |
8.7 |
3.8 |
3.2 |
2.8 |
3.0 |
n/a |
n/a |
n/a |
n/a |
|
Total protein (%) |
3.4 |
4.4 |
5.5 |
3.7 |
1.8 |
3.3 |
2.6 |
1.0 |
0.5 |
0.6 |
|
Casein:whey |
82:18 |
82:18 |
76:24 |
78:22 |
52:48 |
73:27–76:24 |
n/a |
n/a |
n/a |
n/a |
|
Lactose (%) |
4.8 |
4.8 |
4.8 |
4.5 |
6.9 |
4.3 |
n/a |
n/a |
n/a |
n/a |
|
Galactose (%) |
4.0 |
3.3 |
0.3 |
0.6 |
<0.1 |
<0.1 |
n/a |
n/a |
n/a |
n/a |
|
GI (0–100) |
27–37 |
- |
- |
- |
89.3 (donkey) |
- |
31–37 |
69 |
79–92 |
49–64 |
|
Energy (kJ) |
316.9–373.0 |
345.0 |
593.2 |
301.8 |
184.2–205.1 |
328.3 |
179.9 |
195.8 |
225.9 |
126.8 |
|
Calcium (mg) |
119.8 |
183.9 |
181.7 |
130.4 |
92.9 |
106.0 |
113.0 |
120.0 |
118.0 |
160.0 |
|
Potassium (mg) |
145.0 |
101.6 |
120.0 |
181.0 |
50.5 |
156.0 |
122.0 |
162.0 |
27.0 |
67.0 |
Data obtained and collated from a range of supermarket product labels and/or the following sources in the literature [17,22,28,29,34,35,51–58]. Equine milk refers to mare milk unless otherwise specified. n.d., not detected.
The unique classification of AAs and FAs composing individual milks has also sparked interest in the literature. Total and essential AA content are the highest in sheep and reindeer milk, whilst goat, buffalo, and yak milk still possess a higher composition than cow milk [22]. Regarding lipid profile, goat, sheep, and camel milk marginally surpass cow milk concentrations of monounsaturated (MUFA) and polyunsaturated (PUFA) FAs [41]. More notably, goat and sheep milk are considerable sources of short- and medium-chain triglycerides (MCTs), compared to long-chain triglyceride (LCT)-rich cow milk [42,43]. In exemplification of this, three MCTs (caproic acid, caprylic acid, and capric acid) even owe their names to the caprine species (i.e., goats). MCT content (C6:0–C12:0) (as a percentage of total fat content) is the highest in goat (23.0%) and sheep (21.8%) milk, followed by mare (15.2%), cow (10.5%) and human (7.3%) milk [37]. Potentially of further bioactive utility, the content of CLA (a PUFA found in animal milk) and vaccenic acid (a CLA precursor) is also higher in sheep milk (1.2% of total FAs) than cow (0.7%) and goat (0.6%) milk [44]. Finally, the oligosaccharide content of goat milk is approximately five and ten times greater than that of cow and sheep milk, respectively [45].
Beyond differences between mammalian milks, disparities between animal- and plant-sourced milks are more prominent still. The gross protein content of non-dairy milk beverages is typically only half that of cow milk [46]. Nonetheless, soy protein possesses a greater content of branched chain amino acids (BCAAs) than whey protein [47]. However, when using a reference evaluative means for assessing dietary protein quality in humans, the digestible indispensable amino acid score (DIAAS) [48] for milk protein is greater than that of soy protein [49]. Specifically, whilst cow milk protein possesses a DIAAS value ≥118% for all indispensable AAs, soy protein is limited by methionine and cysteine content with a lowest DIAAS of 90.6% despite having a DIAAS value >100% for most individual indispensable AAs [49,50]. Aside from protein content and quality, plant-based milk substitutes, except coconut milk, have lower levels of saturated fatty acids (SFAs) and greater levels of PUFAs and MUFAs than cow milk [46].
The literature has detailed the potential value of, inter alia, protein [59], particularly functional AAs [60]; MUFAs [61]; PUFAs [62]; MCTs [63]; and CLA [15] for improved cardiometabolic health. Hence, given the compositional differences outlined above, a robust assessment of the therapeutic potential of cow milk alternatives is required. The relationship between cow milk and glycaemia, blood pressure, and lipidaemia has been well-researched and reviewed in the literature [4], albeit with variable findings, yet investigations into how alternatively-derived milks influence acute postprandial and chronic fasting metabolism are limited. With a newfound importance emphasising the functional implications of individual foods and beverages for health, a review of non-bovine milk consumption is overdue.
3.2. Digestibility
Before progressing to any disparate effects of milk origin on cardiometabolic health, it is imperative to acknowledge that the protein and fat composition of different milks might also be variably digested. For example, the extent of gastric casein coagulation (or curd formation) alters the absorption of AAs, hence a higher ingested protein load may not necessarily translate to a higher delivered protein load. The acidification of αS1-casein in cow milk forms rigid and durable curds which are difficult to digest, whereas the coagula from goat, camel and mare milk are far more assimilable [22,64]. The degradation of β-lg also varies between species’ milk [22]. Goat [65] and sheep [66] β-lg is digested more efficiently than cow β-lg, but not as rapidly as mare β-lg [67]. Regarding lipid digestion, lipid globule size and FA chain length are two factors that may be inversely related to digestibility, thus influencing fat assimila[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146][147][148][149][150][151][152][153][154][155][156][157][158][159][160][161][162][163][164][165][166][167][168][169][170][171][172][173][174][175][176][177][178][179][180][181][182][183][184][185][186][187][188][189][190][191][192][193][194][195][196][197][198][199][200][201][202][203]tion. Camel and goat milk are noted for small milk fat globules (MFG) compared to sheep, cow, and, most largely, buffalo milk with a mean diameter of 2.99 µm, 3.20 µm, 3.76 µm, 3.78 µm, and 8.7 µm, respectively [68]. However, the direct clinical consequences of MFG size for human health is still largely unknown, with a lack of long-term RCTs having been conducted [69]. Moreover, significant variation in mean MFG size with breed, herd, days in milk, season, and milking period has been reported [70,71]. Hence, this variability within each individual species’ milk devalues current comparisons of MFG size between different species’ milk. Finally, as the ester bonds of short- and medium-chain FAs are more readily hydrolysed than long-chain FAs, a greater fraction of the former, as found in goat and sheep milk, may contribute to higher digestibility [72].
4. The Impact of Milk Origin on Biomarkers of Cardiometabolic Health
4.1. Effects of Milk Origin on Energy Balance & Obesity
4.1.1. Appetite Regulation
4.1.2. Energy Expenditure
4.1.3 Nutrient Processing-Substrate Utilisation and Metabolic Efficiency
4.1.4. Body Weight and Composition
5. Effects of Milk Origin on Insulinaemia, Glycaemia, and Type II Diabetes
5.1. Insulinaemia
5.2. Glycaemia
5.3. Type II Diabetes
6. Effects of Milk Origin on Cardiovascular Health
7. Conclusions
The effect of milk origin on cardiometabolic health is an emerging area of research. There is some data, although primarily from compositional analyses [35,37], in vitro studies [83], animal studies [80], and acute clinical RCTs [77,78,187], that milk from non-bovine origin (notably sheep and goat milk) could prove to be a viable substitute to cow milk for the maintenance, or even enhancement, of cardiometabolic health. However, a collation of the compositional differences and postulated therapeutic utility, as presented in this review, indicate that the level of evidence required to form nutritional recommendations surrounding milk origin is currently lacking. Nonetheless, there are some interesting results, albeit largely from preliminary studies, that have generated excitement around sheep milk consumption for the possible attenuation of cardiometabolic risk. This interest is largely based upon its favourable profile of lipids (e.g., MCTs, CLA), protein (e.g., leucine), and minerals (e.g., calcium). In theory, these compounds could provide protection from obesity, T2D, and CVD through the modulation of postprandial glycaemia, lipidaemia and aminoacidaemia; nutrient processing; postprandial thermogenesis; and/or appetite. Comparably, with desirable nutritional compositions and some promising early findings, goat and buffalo milk may also prove to be robust alternatives to cow milk. However, as with sheep milk, there is currently a stark absence of high-quality research in humans. Hence, as remains pertinent for cow milk, to substantiate any claims that the consumption of cow-milk alternatives can improve cardiometabolic health, causal data from long-term clinical RCTs, ideally with T2D and/or CVD events as the primary endpoint, are required. Evidence from large-scale studies that support the conjectures formed in this review could not only be of value to individuals allergic or intolerant to cow milk, but potentially also to those at an increased risk of cardiometabolic disease. Thus, this review concludes that further exploration into the therapeutic potential of milk beyond the realms of cow dairy is warranted.
This entry is adapted from the peer-reviewed paper 10.3390/nu14020290
References
- Pereira, M.A.; Jacobs, D.R., Jr.; Van Horn, L.; Slattery, M.L.; Kartashov, A.I.; Ludwig, D.S. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA Study. JAMA 2002, 287, 2081–2089. https://doi.org/10.1001/jama.287.16.2081.
- McGregor, R.A.; Poppitt, S.D. Milk protein for improved metabolic health: A review of the evidence. Nutr. Metab. 2013, 10, 46. https://doi.org/10.1186/1743-7075-10-46.
- Lu, L.; Chen, C.; Zhu, J.; Tang, W.; Jacobs, D.R.; Shikany, J.M.; Kahe, K. Calcium Intake Is Inversely Related to Risk of Obe-sity among American Young Adults over a 30-Year Follow-Up. J. Nutr. 2021, 151, 2383–2389. https://doi.org/10.1093/jn/nxab114.
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. https://doi.org/10.3402/fnr.v60.32527.
- Poppitt, S.D. Cow’s Milk and Dairy Consumption: Is There Now Consensus for Cardiometabolic Health? Front. Nutr. 2020, 7, 574725. https://doi.org/10.3389/fnut.2020.574725.
- Acheson, K.J.; Blondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Emady-Azar, S.; Ammon-Zufferey, C.; Monnard, I.; Pinaud, S.; Nielsen-Moennoz, C.; Bovetto, L. Protein choices targeting thermogenesis and metabolism. Am. J. Clin. Nutr. 2011, 93, 525–534. https://doi.org/10.3945/ajcn.110.005850.
- Karst, H.; Steiniger, J.; Noack, R.; Steglich, H.D. Diet-induced thermogenesis in man: Thermic effects of single proteins, carbohydrates and fats depending on their energy amount. Ann. Nutr. Metab. 1984, 28, 245–252. https://doi.org/10.1159/000176811.
- Lorenzen, J.; Frederiksen, R.; Hoppe, C.; Hvid, R.; Astrup, A. The effect of milk proteins on appetite regulation and di-et-induced thermogenesis. Eur. J. Clin. Nutr. 2012, 66, 622–627. https://doi.org/10.1038/ejcn.2011.221.
- Harper, A.; James, A.; Flint, A.; Astrup, A. Increased satiety after intake of a chocolate milk drink compared with a car-bonated beverage, but no difference in subsequent ad libitum lunch intake. Br. J. Nutr. 2007, 97, 579–583. https://doi.org/10.1017/S0007114507339846.
- Veldhorst, M.A.; Nieuwenhuizen, A.G.; Hochstenbach-Waelen, A.; van Vught, A.J.; Westerterp, K.R.; Engelen, M.P.; Brummer, R.J.; Deutz, N.E.; Westerterp-Plantenga, M.S. Dose-dependent satiating effect of whey relative to casein or soy. Physiol. Behav. 2009, 96, 675–682. https://doi.org/10.1016/j.physbeh.2009.01.004.
- Gilbert, J.A.; Joanisse, D.R.; Chaput, J.P.; Miegueu, P.; Cianflone, K.; Almeras, N.; Tremblay, A. Milk supplementation facil-itates appetite control in obese women during weight loss: A randomised, single-blind, placebo-controlled trial. Br. J. Nutr. 2011, 105, 133–143. https://doi.org/10.1017/S0007114510003119.
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein peptides with angiotensin I-converting enzyme inhibitory (ACEI) activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. https://doi.org/10.1080/10408390802304198.
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. https://doi.org/10.1016/j.nut.2013.10.011.
- Sjogren, P.; Rosell, M.; Skoglund-Andersson, C.; Zdravkovic, S.; Vessby, B.; de Faire, U.; Hamsten, A.; Hellenius, M.L.; Fisher, R.M. Milk-derived fatty acids are associated with a more favorable LDL particle size distribution in healthy men. J. Nutr. 2004, 134, 1729–1735. https://doi.org/10.1093/jn/134.7.1729.
- Whigham, L.D.; Watras, A.C.; Schoeller, D.A. Efficacy of conjugated linoleic acid for reducing fat mass: A meta-analysis in humans. Am. J. Clin. Nutr. 2007, 85, 1203–1211. https://doi.org/10.1093/ajcn/85.5.1203.
- Frost, G.; Leeds, A.A.; Dore, C.J.; Madeiros, S.; Brading, S.; Dornhorst, A. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet 1999, 353, 1045–1048. https://doi.org/10.1016/s0140-6736(98)07164-5.
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. https://doi.org/10.1093/ajcn/nqab233.
- Maersk, M.; Belza, A.; Holst, J.J.; Fenger-Gron, M.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Satiety scores and satiety hor-mone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: A controlled trial. Eur. J. Clin. Nutr. 2012, 66, 523–529. https://doi.org/10.1038/ejcn.2011.223.
- Iso, H.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.; Hennekens, C.H.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke 1999, 30, 1772–1779. https://doi.org/10.1161/01.str.30.9.1772.
- Ma, J.; Folsom, A.R.; Melnick, S.L.; Eckfeldt, J.H.; Sharrett, A.R.; Nabulsi, A.A.; Hutchinson, R.G.; Metcalf, P.A. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: The ARIC study. Atherosclerosis Risk in Communities Study. J. Clin. Epidemiol. 1995, 48, 927–940. https://doi.org/10.1016/0895-4356(94)00200-a.
- Massey, L.K. Dairy food consumption, blood pressure and stroke. J. Nutr. 2001, 131, 1875–1878. https://doi.org/10.1093/jn/131.7.1875.
- Claeys, W.; Verraes, C.; Cardoen, S.; De Block, J.; Huyghebaert, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of raw or heated milk from different species: An evaluation of the nutritional and potential health benefits. Food Control. 2014, 42, 188–201.
- Pfeuffer, M.; Schrezenmeir, J. Milk and the metabolic syndrome. Obes. Rev. 2007, 8, 109–118. https://doi.org/10.1111/j.1467-789X.2006.00265.x.
- FAOSTAT. Food & Agriculture Organization of the United Nations, FAOSTAT Statistics Database. 2018. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 6 January 2021).
- Itan, Y.; Jones, B.L.; Ingram, C.J.; Swallow, D.M.; Thomas, M.G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 2010, 10, 36. https://doi.org/10.1186/1471-2148-10-36.
- Bolin, T.D.; Crane, G.G.; Davis, A.E. Lactose intolerance in various ethnic groups in South-East Asia. Australas. Ann. Med. 1968, 17, 300–306. https://doi.org/10.1111/imj.1968.17.4.300.
- Sahi, T. Genetics and epidemiology of adult-type hypolactasia. Scand. J. Gastroenterol. Suppl. 1994, 202, 7–20. https://doi.org/10.3109/00365529409091740.
- Park, Y.W. Overview of Bioactive Components in Milk and Dairy Products. In Bioactive Components in Milk and Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2009, pp 3-12
- Balthazar, C.F.; Pimentel, T.C.; Ferrao, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food De-velopment. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. https://doi.org/10.1111/1541-4337.12250.
- El-Agamy, E.I.; Nawar, M.; Shamsia, S.M.; Awad, S.; Haenlein, G.F. Are camel milk proteins convenient to the nutrition of cow milk allergic children? Small Rumin. Res. 2009, 82, 1–6.
- Sheehan, W.J.; Phipatanakul, W. Tolerance to water buffalo milk in a child with cow milk allergy. Ann. Allergy Asthma. Immunol. 2009, 102, 349. https://doi.org/10.1016/S1081-1206(10)60342-0.
- Carroccio, A.; Cavataio, F.; Montalto, G.; D’Amico, D.; Alabrese, L.; Iacono, G. Intolerance to hydrolysed cow’s milk pro-teins in infants: Clinical characteristics and dietary treatment. Clin. Exp. Allergy 2000, 30, 1597–1603. https://doi.org/10.1046/j.1365-2222.2000.00925.x.
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. https://doi.org/10.1007/s13197-016-2328-3.
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2012, 92, 445–474. https://doi.org/10.1002/jsfa.4690.
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional value and technological suitability of milk from vari-ous animal species used for dairy production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302.
- Pietrzak-Fiećko, R.; Tomczyński, R.; Smoczyński, S.S. Effect of lactation period on the fatty acid composition in mares’ milk from different breeds. Arch. Anim. Breed. 2013, 56, 335–343.
- Pietrzak-Fiecko, R.; Kamelska-Sadowska, A.M. The Comparison of Nutritional Value of Human Milk with other Mam-mals’ Milk. Nutrients 2020, 12, 1404. https://doi.org/10.3390/nu12051404.
- Pietrzak-Fiecko, R. Relationship between the Content of Chlorinated Hydrocarbons and Fatty Acid Composition of Milk Fat. J. Vet. Res. 2018, 62, 71–78. https://doi.org/10.1515/jvetres-2018-0010.
- Zervas, G.; Tsiplakou, E. Goat milk. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 498–518.
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein proteins: Structural and functional aspects. Milk Proteins–From Structure to Biolog-ical Properties and Health Aspects; IntechOpen: Rijeka, Hrvatska, 2016; pp. 1–17.
- Haenlein, G. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163.
- Chilliard, Y.; Rouel, J.; Ferlay, A.; Bernard, L.; Gaborit, P.; Raynal-Ljutovac, K.; Lauret, A.; Leroux, C. Optimising goat’s milk and cheese fatty acid composition. In Improving the Fat Content of Foods; Elsevier: Amsterdam, The Netherlands, 2006; pp. 281–312.
- MacGibbon, A.; Taylor, M. Composition and structure of bovine milk lipids. In Advanced Dairy Chemistry Volume 2 Lipids; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–42.
- Jahreis, G.; Fritsche, J.; Kraft, J. Species-dependent, seasonal, and dietary variation of conjugated linoleic acid in milk. Adv. Conjug. Linoleic Acid Res. 1999, 1, 215–225.
- Martinez-Ferez, A.; Rudloff, S.; Guadix, A.; Henkel, C.A.; Pohlentz, G.; Boza, J.J.; Guadix, E.M.; Kunz, C. Goats’ milk as a natural source of lactose-derived oligosaccharides: Isolation by membrane technology. Int. Dairy J. 2006, 16, 173–181.
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alter-natives. Int. Dairy J. 2018, 87, 84–92.
- Rde, C.A.; Bressan, J.; Paiva, A.C. Effects of protein quality on appetite and energy metabolism in normal weight subjects. Arq. Bras. Endocrinol. Metab. 2010, 54, 45–51. https://doi.org/10.1590/s0004-27302010000100008.
- FAO. Dietary Protein Quality Evaluation. In Proceedings of the FAO Food and Nutrition, Rome, Italy, 31 March–2 April 2011.
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. https://doi.org/10.3945/jn.114.195438.
- Singhal, S.; Baker, R.D.; Baker, S.S. A Comparison of the Nutritional Value of Cow’s Milk and Nondairy Beverages. J. Pedi-atr. Gastroenterol. Nutr. 2017, 64, 799–805. https://doi.org/10.1097/MPG.0000000000001380.
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.; Jiménez-Munguía, M.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2021, 1–32.
- Abd El-Salam, M.H.; El-Shibiny, S. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci. Technol. 2011, 91, 663–699.
- Sawaya, W.; Khalil, J.; Al-Shalhat, A.; Al-Mohammad, H. Chemical composition and nutritional quality of camel milk. J. Food Sci. 1984, 49, 744–747.
- MS Gorban, A.; Izzeldin, O.M. Fatty acids and lipids of camel milk and colostrum. Int. J. Food Sci. Nutr. 2001, 52, 283–287.
- Jahreis, G.; Fritsche, J.; Möckel, P.; Schöne, F.; Möller, U.; Steinhart, H. The potential anticarcinogenic conjugated linoleic acid, cis-9, trans-11 C18: 2, in milk of different species: Cow, goat, ewe, sow, mare, woman. Nutr. Res. 1999, 19, 1541–1549.
- Welsch, U.; Buchheim, W.; Schumacher, U.; Schinko, I.; Patton, S. Structural, histochemical and biochemical observations on horse milk-fat-globule membranes and casein micelles. Histochemistry 1988, 88, 357–365.
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s milk substitutes for children: Nutritional aspects of milk from different mammalian species, special formula and plant-based beverages. Nutrients 2019, 11, 1739.
- Innocente, N.; Parpinel, M.; Rinaldi, A.; Biasutti, M. 4.8.(S4. 36) Composition and Nutritional Value of Donkey Milk. Bull. Int. Dairy Fed. 2012, 1201, 168.
- Westerterp-Plantenga, M.S.; Lemmens, S.G.; Westerterp, K.R. Dietary protein-its role in satiety, energetics, weight loss and health. Br. J. Nutr. 2012, 108 (Suppl. 2), S105–S112. https://doi.org/10.1017/S0007114512002589.
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. https://doi.org/10.1007/s00726-013-1500-6.
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syn-drome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. https://doi.org/10.1007/s11745-010-3524-y.
- Tsitouras, P.D.; Gucciardo, F.; Salbe, A.D.; Heward, C.; Harman, S.M. High omega-3 fat intake improves insulin sensitivi-ty and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm. Metab. Res. 2008, 40, 199–205. https://doi.org/10.1055/s-2008-1046759.
- St-Onge, M.P.; Jones, P.J. Physiological effects of medium-chain triglycerides: Potential agents in the prevention of obesity. J. Nutr. 2002, 132, 329–332. https://doi.org/10.1093/jn/132.3.329.
- Ceballos, L.S.; Morales, E.R.; Martinez, L.P.; Extremera, F.G.; Sampelayo, M.R. Utilization of nitrogen and energy from di-ets containing protein and fat derived from either goat milk or cow milk. J. Dairy Res. 2009, 76, 497–504. https://doi.org/10.1017/S0022029909990252.
- Michaelidou, A. Factors influencing nutritional and health profile of milk and milk products. Small Rumin. Res. 2008, 79, 42–50.
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Métro, F.; Haertle, T.; Chobert, J.-M. Peptic hydrolysis of ovine β-lactoglobulin and α-lactalbumin Exceptional susceptibility of native ovine β-lactoglobulin to pepsinolysis. Int. Dairy J. 2005, 15, 17–27.
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Compari-son of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563.
- El-Zeini, H.M. Microstructure, rheological and geometrical properties of fat globules of milk from different animal species. Pol. J. Food Nutr. Sci. 2006, 56, 147–154.
- Argov-Argaman, N. Symposium review: Milk fat globule size: Practical implications and metabolic regulation. J. Dairy Sci. 2019, 102, 2783–2795. https://doi.org/10.3168/jds.2018-15240.
- Park, Y.; Juárez, M.; Ramos, M.; Haenlein, G. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. https://doi.org/10.1016/j.smallrumres.2006.09.013
- Fleming, A.; Schenkel, F.; Chen, J.; Malchiodi, F.; Ali, R.; Mallard, B.; Sargolzaei, M.; Corredig, M.; Miglior, F. Variation in fat globule size in bovine milk and its prediction using mid-infrared spectroscopy. J. Dairy Sci. 2017, 100, 1640–1649.
- Park, Y. Hypo-allergenic and therapeutic significance of goat milk. Small Rumin. Res. 1994, 14, 151–159.
- Lejeune, M.P.; Kovacs, E.M.; Westerterp-Plantenga, M.S. Additional protein intake limits weight regain after weight loss in humans. Br. J. Nutr. 2005, 93, 281–289. https://doi.org/10.1079/bjn20041305.
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy ex-penditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. https://doi.org/10.3945/an.113.003723.
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein source, quantity, and time of consumption determine the ef-fect of proteins on short-term food intake in young men. J. Nutr. 2004, 134, 3011–3015. https://doi.org/10.1093/jn/134.11.3011.
- Baer, D.J.; Stote, K.S.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Clevidence, B.A. Whey protein but not soy protein supple-mentation alters body weight and composition in free-living overweight and obese adults. J. Nutr. 2011, 141, 1489–1494. https://doi.org/10.3945/jn.111.139840.
- Rubio-Martin, E.; Garcia-Escobar, E.; Ruiz de Adana, M.S.; Lima-Rubio, F.; Pelaez, L.; Caracuel, A.M.; Bermudez-Silva, F.J.; Soriguer, F.; Rojo-Martinez, G.; Olveira, G. Comparison of the Effects of Goat Dairy and Cow Dairy Based Breakfasts on Satiety, Appetite Hormones, and Metabolic Profile. Nutrients 2017, 9, 877. https://doi.org/10.3390/nu9080877.
- Milan, A.M.; Hodgkinson, A.J.; Mitchell, S.M.; Prodhan, U.K.; Prosser, C.G.; Carpenter, E.A.; Fraser, K.; Cameron-Smith, D. Digestive Responses to Fortified Cow or Goat Dairy Drinks: A Randomised Controlled Trial. Nutrients 2018, 10, 1492. https://doi.org/10.3390/nu10101492.
- Alferez, M.J.; Barrionuevo, M.; Lopez Aliaga, I.; Sanz-Sampelayo, M.R.; Lisbona, F.; Robles, J.C.; Campos, M.S. Digestive utilization of goat and cow milk fat in malabsorption syndrome. J. Dairy Res. 2001, 68, 451–461. https://doi.org/10.1017/s0022029901004903.
- Cano, M.P.G; Van Nieuwenhove, C.; Chaila, Z.; Bazan, C.; Gonzalez, S. Effects of short-term mild calorie restriction diet and renutrition with ruminant milks on leptin levels and other metabolic parameters in mice. Nutrition 2009, 25, 322–329. https://doi.org/10.1016/j.nut.2008.09.003.
- Roh, C.; Han, J.; Tzatsos, A.; Kandror, K.V. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E322–E330. https://doi.org/10.1152/ajpendo.00230.2002.
- Mars, M.; de Graaf, C.; de Groot, C.P.; van Rossum, C.T.; Kok, F.J. Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet. Int. J. Obes. 2006, 30, 122–128. https://doi.org/10.1038/sj.ijo.0803070.
- Sanchez-Moya, T.; Planes-Munoz, D.; Frontela-Saseta, C.; Ros-Berruezo, G.; Lopez-Nicolas, R. Milk whey from different animal species stimulates the in vitro release of CCK and GLP-1 through a whole simulated intestinal digestion. Food Funct. 2020, 11, 7208–7216. https://doi.org/10.1039/d0fo00767f.
- Uchida, M.; Ohshiba, Y.; Mogami, O. Novel dipeptidyl peptidase-4-inhibiting peptide derived from beta-lactoglobulin. J. Pharmacol. Sci. 2011, 117, 63–66. https://doi.org/10.1254/jphs.11089sc.
- Tulipano, G.; Cocchi, D.; Caroli, A.M. Comparison of goat and sheep β-lactoglobulin to bovine β-lactoglobulin as poten-tial source of dipeptidyl peptidase IV (DPP-4) inhibitors. Int. Dairy J. 2012, 24, 97–101.
- Vargas-Bello-Perez, E.; Marquez-Hernandez, R.I.; Hernandez-Castellano, L.E. Bioactive peptides from milk: Animal de-terminants and their implications in human health. J. Dairy Res. 2019, 86, 136–144. https://doi.org/10.1017/S0022029919000384.
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. https://doi.org/10.1080/07315724.2007.10719651.
- Seaton, T.B.; Welle, S.L.; Warenko, M.K.; Campbell, R.G. Thermic effect of medium-chain and long-chain triglycerides in man. Am. J. Clin. Nutr. 1986, 44, 630–634. https://doi.org/10.1093/ajcn/44.5.630.
- Scalfi, L.; Coltorti, A.; Contaldo, F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. Am. J. Clin. Nutr. 1991, 53, 1130–1133. https://doi.org/10.1093/ajcn/53.5.1130.
- Matsuo, T.; Matsuo, M.; Taguchi, N.; Takeuchi, H. The thermic effect is greater for structured medium- and long-chain triacylglycerols versus long-chain triacylglycerols in healthy young women. Metabolism 2001, 50, 125–130. https://doi.org/10.1053/meta.2001.18571.
- Dulloo, A.G.; Fathi, M.; Mensi, N.; Girardier, L. Twenty-four-hour energy expenditure and urinary catecholamines of hu-mans consuming low-to-moderate amounts of medium-chain triglycerides: A dose-response study in a human respira-tory chamber. Eur. J. Clin. Nutr. 1996, 50, 152–158.
- Hill, J.O.; Peters, J.C.; Yang, D.; Sharp, T.; Kaler, M.; Abumrad, N.N.; Greene, H.L. Thermogenesis in humans during over-feeding with medium-chain triglycerides. Metabolism 1989, 38, 641–648. https://doi.org/10.1016/0026-0495(89)90101-7.
- Matsuo, T.; Takeuchi, H. Effects of structured medium- and long-chain triacylglycerols in diets with various levels of fat on body fat accumulation in rats. Br. J. Nutr. 2004, 91, 219–225. https://doi.org/10.1079/BJN20031041.
- Lasekan, J.B.; Rivera, J.; Hirvonen, M.D.; Keesey, R.E.; Ney, D.M. Energy expenditure in rats maintained with intravenous or intragastric infusion of total parenteral nutrition solutions containing medium- or long-chain triglyceride emulsions. J. Nutr. 1992, 122, 1483–1492. https://doi.org/10.1093/jn/122.7.1483.
- Posati, L.P.; Orr, M.L. Composition of Foods—Dairy and Egg Products: Raw, Processed, Prepared; Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 1976.
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. https://doi.org/10.1073/pnas.94.26.14930.
- Ravussin, E.; Lillioja, S.; Anderson, T.E.; Christin, L.; Bogardus, C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J. Clin. Investig. 1986, 78, 1568–1578. https://doi.org/10.1172/JCI112749.
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. https://doi.org/10.1093/jn/133.1.261S.
- Smilowitz, J.T.; Dillard, C.J.; German, J.B. Milk beyond essential nutrients: The metabolic food. Aust. J. Dairy Technol. 2005, 60, 77.
- Dulloo, A.G. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883.
- Lopez-Aliaga, I.; Alferez, M.J.; Barrionuevo, M.; Nestares, T.; Sanz Sampelayo, M.R.; Campos, M.S. Study of nutritive uti-lization of protein and magnesium in rats with resection of the distal small intestine. Beneficial effect of goat milk. J. Dairy Sci. 2003, 86, 2958–2966. https://doi.org/10.3168/jds.S0022-0302(03)73893-4.
- Singh, M.; Sharma, R.; Ranvir, S.; Gandhi, K.; Mann, B. Profiling and distribution of minerals content in cow, buffalo and goat milk. Indian J. Dairy Sci. 2019, 72, 480–488.
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. https://doi.org/10.1007/s00198-017-4230-x.
- Davies, K.M.; Heaney, R.P.; Recker, R.R.; Lappe, J.M.; Barger-Lux, M.J.; Rafferty, K.; Hinders, S. Calcium intake and body weight. J. Clin. Endocrinol. Metab. 2000, 85, 4635–4638. https://doi.org/10.1210/jcem.85.12.7063.
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138.
- Zemel, M.B.; Richards, J.; Milstead, A.; Campbell, P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes. Res. 2005, 13, 1218–1225. https://doi.org/10.1038/oby.2005.144.
- Booth, A.O.; Huggins, C.E.; Wattanapenpaiboon, N.; Nowson, C.A. Effect of increasing dietary calcium through supple-ments and dairy food on body weight and body composition: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1013–1025. https://doi.org/10.1017/S0007114515001518.
- Mohammad, M.A.; Sunehag, A.L.; Rodriguez, L.A.; Haymond, M.W. Galactose promotes fat mobilization in obese lactat-ing and nonlactating women. Am. J. Clin. Nutr. 2011, 93, 374–381. https://doi.org/10.3945/ajcn.110.005785.
- Charrière, N.; Montani, J.-P.; Dulloo, A.G. Postprandial thermogenesis and respiratory quotient in response to galactose: Comparison with glucose and fructose in healthy young adults. J. Nutr. Sci. 2016, 5, e4.
- Young, J.B.; Weiss, J.; Boufath, N. Effects of dietary monosaccharides on sympathetic nervous system activity in adipose tissues of male rats. Diabetes 2004, 53, 1271–1278. https://doi.org/10.2337/diabetes.53.5.1271.
- Goseki-Sone, M.; Maruyama, R.; Sogabe, N.; Hosoi, T. Effects of dietary lactose on long-term high-fat-diet-induced obesity in rats. Obesity 2007, 15, 2605–2613. https://doi.org/10.1038/oby.2007.312.
- Cataldi, T.R.; Angelotti, M.; Bianco, G. Determination of mono-and disaccharides in milk and milk products by high-performance anion-exchange chromatography with pulsed amperometric detection. Anal. Chim. Acta 2003, 485, 43–49.
- Mack, P. A preliminary nutrition study of the value of goat’s milk in the diet of children. Yearb. Am. Goat Soc. 1952.
- Razafindrakoto, O.; Ravelomanana, N.; Rasolofo, A.; Rakotoarimanana, R.D.; Gourgue, P.; Coquin, P.; Briend, A.; Desjeux, J.F. Goat’s milk as a substitute for cow’s milk in undernourished children: A randomized double-blind clinical trial. Pedi-atrics 1994, 94, 65–69.
- Guo, H.Y.; Pang, K.; Zhang, X.Y.; Zhao, L.; Chen, S.W.; Dong, M.L.; Ren, F.Z. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 2007, 90, 1635–1643. https://doi.org/10.3168/jds.2006-600.
- Park, Y. Rheological characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 73–87. https://doi.org/10.1016/j.smallrumres.2006.09.015
- Shamsia, S. Nutritional and therapeutic properties of camel and human milks. Int. J. Genet. Mol. Biol. 2009, 1, 052–058.
- Kanwal, R.; Ahmed, T.; Mirza, B. Comparative analysis of quality of milk collected from buffalo, cow, goat and sheep of Rawalpindi/Islamabad region in Pakistan. Asian J. Plant Sci. 2004, 3, 300–305.
- Barłowska, J. Nutritional value and technological usability of milk from cows of 7 breeds maintained in Poland. Post-doctoral Thesis, Agriculture Academy, University of Life Sciences, Lublin, Poland, 2007.
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11.
- Park, Y.W.; Mahoney, A.W.; Hendricks, D.G. Bioavailability of iron in goat milk compared with cow milk fed to anemic rats. J. Dairy Sci. 1986, 69, 2608–2615.
- Korish, A.A. The antidiabetic action of camel milk in experimental type 2 diabetes mellitus: An overview on the changes in incretin hormones, insulin resistance, and inflammatory cytokines. Horm. Metab. Res. 2014, 46, 404–411. https://doi.org/10.1055/s-0034-1368711.
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underly-ing health benefits for active people (review). J. Nutr. Biochem 2003, 14, 251–258. https://doi.org/10.1016/s0955-2863(03)00030-5.
- Master, P.B.Z.; Macedo, R.C.O. Effects of dietary supplementation in sport and exercise: A review of evidence on milk proteins and amino acids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1225–1239. https://doi.org/10.1080/10408398.2020.1756216.
- Astrup, A. The satiating power of protein—A key to obesity prevention? Am. J. Clin. Nutr. 2005, 82, 1–2. https://doi.org/10.1093/ajcn.82.1.1.
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S-1329S. https://doi.org/10.3945/ajcn.114.084038.
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composi-tion changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. https://doi.org/10.1093/nutrit/nuv065.
- Hansen, T.T.; Astrup, A.; Sjodin, A. Are Dietary Proteins the Key to Successful Body Weight Management? A Systematic Review and Meta-Analysis of Studies Assessing Body Weight Outcomes after Interventions with Increased Dietary Pro-tein. Nutrients 2021, 13, 3193. https://doi.org/10.3390/nu13093193.
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. https://doi.org/10.3390/nu11081825.
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2020, 59, 19–34. https://doi.org/10.1007/s00394-019-01936-3.
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. https://doi.org/10.3945/jn.114.204305.
- Poppitt, S.D. Milk proteins and human health. In Milk Proteins, Elsevier: Amsterdam, The Netherlands, 2020; pp. 651–669.
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382.
- Recio, I.; de la Fuente, M.A.; Juárez, M.; Ramos, M. Bioactive components in sheep milk. In Bioactive Components in Milk and Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 83–104.
- Poppitt, S.D.; Strik, C.M.; MacGibbon, A.K.; McArdle, B.H.; Budgett, S.C.; McGill, A.T. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol. Behav. 2010, 101, 161–167. https://doi.org/10.1016/j.physbeh.2010.04.036.
- Kaviani, S.; Cooper, J.A. Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. Eur. J. Clin. Nutr. 2017, 71, 1154–1165. https://doi.org/10.1038/ejcn.2016.250.
- Park, Y.; Storkson, J.M.; Albright, K.J.; Liu, W.; Pariza, M.W. Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 1999, 34, 235–241.
- Belury, M.A. Dietary conjugated linoleic acid in health: Physiological effects and mechanisms of action. Annu. Rev. Nutr. 2002, 22, 505–531. https://doi.org/10.1146/annurev.nutr.22.021302.121842.
- Belury, M.A.; Mahon, A.; Banni, S. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J. Nutr. 2003, 133, 257S–260S. https://doi.org/10.1093/jn/133.1.257S.
- Chin, S.; Liu, W.; Storkson, J.; Ha, Y.; Pariza, M. Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J. Food Compos. Anal. 1992, 5, 185–197.
- Larsen, T.M.; Toubro, S.; Astrup, A. Efficacy and safety of dietary supplements containing CLA for the treatment of obesi-ty: Evidence from animal and human studies. J. Lipid Res. 2003, 44, 2234–2241. https://doi.org/10.1194/jlr.R300011-JLR200.
- Gaullier, J.M.; Halse, J.; Hoivik, H.O.; Hoye, K.; Syvertsen, C.; Nurminiemi, M.; Hassfeld, C.; Einerhand, A.; O’Shea, M.; Gudmundsen, O. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br. J. Nutr. 2007, 97, 550–560. https://doi.org/10.1017/S0007114507381324.
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. https://doi.org/10.3390/nu11020370.
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolu-tionary origins. Br. J. Nutr. 2008, 99, 931–940. https://doi.org/10.1017/S0007114507853347.
- Zemel, M.B.; Teegarden, D.; Van Loan, M.; Schoeller, D.; Matkovic, V.; Lyle, R.; Craig, B. Role of dairy products in modu-lating weight and fat loss: A multi-center trial. FASEB J. 2004, 18, 4-5.
- Zemel, M.B. Role of calcium and dairy products in energy partitioning and weight management. Am. J. Clin. Nutr. 2004, 79, 907S–912S. https://doi.org/10.1093/ajcn/79.5.907S.
- Nuttall, F.Q.; Gannon, M.C. Quantitative importance of dietary constituents other than glucose as insulin secretagogues in type II diabetes. Diabetes Care 1988, 11, 72–76. https://doi.org/10.2337/diacare.11.1.72.
- Coe, S.; Ryan, L. Impact of polyphenol-rich sources on acute postprandial glycaemia: A systematic review. J. Nutr. Sci. 2016, 5, e24. https://doi.org/10.1017/jns.2016.11.
- Gunnerud, U.; Holst, J.J.; Ostman, E.; Bjorck, I. The glycemic, insulinemic and plasma amino acid responses to equi-carbohydrate milk meals, a pilot- study of bovine and human milk. Nutr. J. 2012, 11, 83. https://doi.org/10.1186/1475-2891-11-83.
- Floyd, J.C., Jr.; Fajans, S.S.; Conn, J.W.; Knopf, R.F.; Rull, J. Stimulation of insulin secretion by amino acids. J. Clin. Investig. 1966, 45, 1487–1502. https://doi.org/10.1172/JCI105456.
- van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. https://doi.org/10.1093/ajcn/72.1.96.
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem 2013, 24, 1–5. https://doi.org/10.1016/j.jnutbio.2012.07.008.
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Bjorck, I.M. Effect of whey on blood glucose and insulin responses to composite break-fast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. https://doi.org/10.1093/ajcn.82.1.69.
- Jakubowicz, D.; Froy, O.; Ahren, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insu-linotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetolo-gia 2014, 57, 1807–1811. https://doi.org/10.1007/s00125-014-3305-x.
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58.
- Song, J.J.; Wang, Q.; Du, M.; Ji, X.M.; Mao, X.Y. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. https://doi.org/10.3168/jds.2016-11828.
- Comerford, K.B.; Pasin, G. Emerging Evidence for the Importance of Dietary Protein Source on Glucoregulatory Markers and Type 2 Diabetes: Different Effects of Dairy, Meat, Fish, Egg, and Plant Protein Foods. Nutrients 2016, 8, 446. https://doi.org/10.3390/nu8080446.
- Sun, L.; Tan, K.W.J.; Han, C.M.S.; Leow, M.K.; Henry, C.J. Impact of preloading either dairy or soy milk on postprandial glycemia, insulinemia and gastric emptying in healthy adults. Eur. J. Nutr. 2015, 56, 77–87. https://doi.org/10.1007/s00394-015-1059-y.
- Sun, L.; Tan, K.W.; Siow, P.C.; Henry, C.J. Soya milk exerts different effects on plasma amino acid responses and incretin hormone secretion compared with cows’ milk in healthy, young men. Br. J. Nutr. 2016, 116, 1216–1221. https://doi.org/10.1017/S0007114516003214.
- Agrawal, R.; Singh, G.; Nayak, K.; Kochar, D.; Sharma, R.; Beniwal, R.; Rastogi, P.; Gupta, R. Prevalence of diabetes in camel-milk consuming Raica rural community of north-west Rajasthan. Int. J. Diab. Dev. Ctries. 2004, 24, 109–114.
- Yagil, R.; Zagorski, O.; Van Creveld, C.; Saran, A. Science and camel’s milk production. In Proceedings of Chameux et dromedaries, animaux laitiers (Dromedaries and Camels, Milking Animals); Ed. Saint Martin, G. Expansion Scientifique Francais, Paris, pp. 75–89.
- Zagorski, O.; Maman, A.; Yaffe, A.; Meisler, A.; Van Creveld, C.; Yagil, R. Insulin in milk-a comparative study. Int. J. Anim. Sci. 1998, 13, 241–244.
- Agrawal, R.P.; Jain, S.; Shah, S.; Chopra, A.; Agarwal, V. Effect of camel milk on glycemic control and insulin requirement in patients with type 1 diabetes: 2-years randomized controlled trial. Eur. J. Clin. Nutr. 2011, 65, 1048–1052. https://doi.org/10.1038/ejcn.2011.98.
- Malik, A.; Al-Senaidy, A.; Skrzypczak-Jankun, E.; Jankun, J. A study of the anti-diabetic agents of camel milk. Int. J. Mol. Med. 2012, 30, 585–592. https://doi.org/10.3892/ijmm.2012.1051.
- Trinchese, G.; Cavaliere, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. https://doi.org/10.3389/fphys.2018.00032.
- Belury, M.; Vanden Heuvel, J. Modulation of diabetes by conjugated linoleic acid. Adv. Conjug. Linoleic Acid Res. 1999, 1, 404–411.
- Li, K.; Sinclair, A.J.; Zhao, F.; Li, D. Uncommon Fatty Acids and Cardiometabolic Health. Nutrients 2018, 10, 1559. https://doi.org/10.3390/nu10101559.
- Riserus, U.; Arner, P.; Brismar, K.; Vessby, B. Treatment with dietary trans10cis12 conjugated linoleic acid causes iso-mer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 2002, 25, 1516–1521. https://doi.org/10.2337/diacare.25.9.1516.
- Ryder, J.W.; Portocarrero, C.P.; Song, X.M.; Cui, L.; Yu, M.; Combatsiaris, T.; Galuska, D.; Bauman, D.E.; Barbano, D.M.; Charron, M.J.; et al. Isomer-specific antidiabetic properties of conjugated linoleic acid. Improved glucose tolerance, skele-tal muscle insulin action, and UCP-2 gene expression. Diabetes 2001, 50, 1149–1157. https://doi.org/10.2337/diabetes.50.5.1149.
- Foster-Powell, K.; Miller, J.B. International tables of glycemic index. Am. J. Clin. Nutr. 1995, 62, 871S–890S. https://doi.org/10.1093/ajcn/62.4.871S.
- Jenkins, D.J.; Wolever, T.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366.
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. https://doi.org/10.1007/s11130-016-0583-0.
- Ercan, N.; Nuttall, F.Q.; Gannon, M.C.; Redmon, J.B.; Sheridan, K.J. Effects of glucose, galactose, and lactose ingestion on the plasma glucose and insulin response in persons with non-insulin-dependent diabetes mellitus. Metabolism 1993, 42, 1560–1567. https://doi.org/10.1016/0026-0495(93)90151-d.
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. https://doi.org/10.3945/ajcn.113.059030.
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. https://doi.org/10.3945/an.112.002030.
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. https://doi.org/10.1007/s11745-010-3393-4.
- Drouin-Chartier, J.P.; Cote, J.A.; Labonte, M.E.; Brassard, D.; Tessier-Grenier, M.; Desroches, S.; Couture, P.; Lamarche, B. Comprehensive Review of the Impact of Dairy Foods and Dairy Fat on Cardiometabolic Risk. Adv. Nutr. 2016, 7, 1041–1051. https://doi.org/10.3945/an.115.011619.
- Greenberger, N.J.; Skillman, T.G. Medium-chain triglycerides. N. Engl. J. Med. 1969, 280, 1045–1058. https://doi.org/10.1056/NEJM196905082801906.
- Paszczyk, B.; Tońska, E.; Łuczyńska, J. Health-promoting value of cow, sheep and goat milk and yogurts. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2019, 69, 182–192.
- Penn, D.; Dolderer, M.; Schmidt-Sommerfeld, E. Carnitine concentrations in the milk of different species and infant for-mulas. Biol. Neonate. 1987, 52, 70–79. https://doi.org/10.1159/000242686.
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629.
- Chan, A.H.; D’Souza, R.F.; Beals, J.W.; Zeng, N.; Prodhan, U.; Fanning, A.C.; Poppitt, S.D.; Li, Z.; Burd, N.A.; Camer-on-Smith, D. The degree of aminoacidemia after dairy protein ingestion does not modulate the postexercise anabolic re-sponse in young men: A randomized controlled trial. J. Nutr. 2019, 149, 1511–1522.
- Mitchell, C.J.; McGregor, R.A.; D’Souza, R.F.; Thorstensen, E.B.; Markworth, J.F.; Fanning, A.C.; Poppitt, S.D.; Camer-on-Smith, D. Consumption of Milk Protein or Whey Protein Results in a Similar Increase in Muscle Protein Synthesis in Middle Aged Men. Nutrients 2015, 7, 8685–8699. https://doi.org/10.3390/nu7105420.
- Weijzen, M.E.G.; van Gassel, R.J.J.; Kouw, I.W.K.; Trommelen, J.; Gorissen, S.H.M.; van Kranenburg, J.; Goessens, J.P.B.; van de Poll, M.C.G.; Verdijk, L.B.; van Loon, L.J.C. Ingestion of Free Amino Acids Compared with an Equivalent Amount of Intact Protein Results in More Rapid Amino Acid Absorption and Greater Postprandial Plasma Amino Acid Availability without Affecting Muscle Protein Synthesis Rates in Young Adults in a Double-Blind Randomized Trial. J. Nutr. 2021. https://doi.org/10.1093/jn/nxab305.
- Sarwar, G.; Botting, H.G.; Davis, T.A.; Darling, P.; Pencharz, P.B. Free amino acids in milks of human subjects, other pri-mates and non-primates. Br. J. Nutr. 1998, 79, 129–131. https://doi.org/10.1079/bjn19980023.
- Jenness, R. Composition and characteristics of goat milk: Review 1968−1979. J. Dairy Sci. 1980, 63, 1605–1630.
- Milan, A.M.; Samuelsson, L.M.; Shrestha, A.; Sharma, P.; Day, L.; Cameron-Smith, D. Circulating Branched Chain Amino Acid Concentrations Are Higher in Dairy-Avoiding Females following an Equal Volume of Sheep Milk Relative to Cow Milk: A Randomized Controlled Trial. Front. Nutr. 2020, 7, 553674. https://doi.org/10.3389/fnut.2020.553674.
- Riserus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. https://doi.org/10.1016/j.plipres.2008.10.002.
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. https://doi.org/10.3168/jds.2016-12224.
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. https://doi.org/10.1016/j.jacc.2020.05.077.
- Salamon, R.; Salamon, S.; Csapó-Kiss, Z.; Csapó, J. Composition of mare’s colostrum and milk I. Fat content, fatty acid composition and vitamin contents. Acta Univ. Sapientiae Aliment. 2009, 2, 119–131.
- Barreto, Í.M.L.G.; Rangel, A.H.d.N.; Urbano, S.A.; Bezerra, J.d.S.; Oliveira, C.A.d.A. Equine milk and its potential use in the human diet. Food Sci. Technol. 2019, 39, 1–7.
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 2001, 37, 1053–1059. https://doi.org/10.1161/01.hyp.37.4.1053.
- Tuomilehto, J.; Lindstrom, J.; Hyyrynen, J.; Korpela, R.; Karhunen, M.L.; Mikkola, L.; Jauhiainen, T.; Seppo, L.; Nissinen, A. Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. J. Hum. Hypertens. 2004, 18, 795–802. https://doi.org/10.1038/sj.jhh.1001745.
- Turpeinen, A.M.; Ikonen, M.; Kivimaki, A.S.; Kautiainen, H.; Vapaatalo, H.; Korpela, R. A spread containing bioactive milk peptides Ile-Pro-Pro and Val-Pro-Pro, and plant sterols has antihypertensive and cholesterol-lowering effects. Food Funct. 2012, 3, 621–627. https://doi.org/10.1039/c2fo10286b.
- Turpeinen, A.M.; Jarvenpaa, S.; Kautiainen, H.; Korpela, R.; Vapaatalo, H. Antihypertensive effects of bioactive tripep-tides-a random effects meta-analysis. Ann. Med. 2013, 45, 51–56. https://doi.org/10.3109/07853890.2012.663926.
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. https://doi.org/10.1128/AEM.69.9.5297-5305.2003.
- Politis, I.; Theodorou, G. Angiotensin I-converting (ACE)-inhibitory and anti-inflammatory properties of commercially available Greek yoghurt made from bovine or ovine milk: A comparative study. Int. Dairy J. 2016, 58, 46–49.
- Murakami, M.; Tonouchi, H.; Takahashi, R.; Kitazawa, H.; Kawai, Y.; Negishi, H.; Saito, T. Structural analysis of a new anti-hypertensive peptide (beta-lactosin B) isolated from a commercial whey product. J. Dairy Sci. 2004, 87, 1967–1974. https://doi.org/10.3168/jds.S0022-0302(04)70013-2.
- Geerlings, A.; Villar, I.; Zarco, F.H.; Sánchez, M.; Vera, R.; Gomez, A.Z.; Boza, J.; Duarte, J. Identification and characteriza-tion of novel angiotensin-converting enzyme inhibitors obtained from goat milk. J. Dairy Sci. 2006, 89, 3326–3335.
- Silva, S.V.; Pihlanto, A.; Malcata, F.X. Bioactive peptides in ovine and caprine cheeselike systems prepared with proteases from Cynara cardunculus. J. Dairy Sci. 2006, 89, 3336–3344. https://doi.org/10.3168/jds.S0022-0302(06)72370-0.
- Morgan, T.; Nowson, C.; Snowden, R.; Teow, B.H.; Hadji, E.; Hodgson, M.; Anderson, A.; Wilson, D.; Adam, W. The effect of sodium potassium, calcium and magnesium on blood pressure. Recent Adv. Clin. Nutr. 1986, 2, 94.
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hyperten-sion. J. Clin. Hypertens. 2008, 10, 3–11. https://doi.org/10.1111/j.1751-7176.2008.08575.x.
