Nanocomposite membranes obtained by dispersing solid particles within a polymeric matrix are referred to as mixed-matrix membranes (MMMs) and are potentially capable to exceed the trade-off restrictions between membrane permeability and selectivity that is an intrinsic trait for polymeric membranes applied to gas separation.
- Mixed Matrix Membranes
- Gas Separation
- Polymer
- Transport
1. Introduction
[1]Various studies showed a good compatibility of the polymers belonging to “Pebax family” with a huge number of fillers for the preparation of nanocomposite membranes to be used in CO2 separation. These researches were reviewed in 2018 by Kardani et al. [2] and in 2022 by Bernardo and Clarizia .
Pebax as host matrix takes advantages of a combined flexibility and mechanical resistance for the presence of PE and PA units: the prevalence of one feature on the other depends on the relative amount of them. In addition, these polymers present intrinsic gas transport properties of interest for the separation of gaseous mixture containing CO2 [3].
Therefore, several promising fillers, capable of enhancing the structural and gas transport properties of the polymers establishing specific interactions with gas molecules, can be favorably incorporated within the host matrix. They include inorganic particles[4], carbon materials [5][6][7][8][9][10], zeolites[11][12]. An emerging trend is represented by 2D nanoparticles [13][14][15]. Other additives were recently explored to improve the separation performance of the Pebax matrix[16][17].
2. Fillers
An overview of the main filler types incorporated in Pebax polymers is reported in Figure 1. Particle type, size, shape and loading are the main factors that influence the MMM performance.
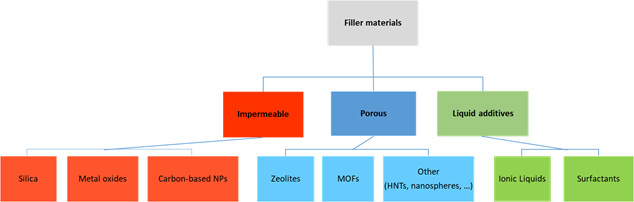
Figure 1. Filler typology used to prepare MMMs based on Pebax polymers[18].
Both porous and non-porous particles can be used in Pebax matrix, whereas the first ones offer additional routes to the permeating gas molecules, according to the opening size of their channels. On the other hand, the blocking of the pores must be absolutely avoided to maintain their greater effectiveness. Also surface properties/sorption properties suffer from this drawback.
In the field of porous fillers, for those having a high aspect ratio, their orientation plays a fundamental role. Significantly different transport rates are observed depending on whether the particles are arranged parallel or perpendicular to the membrane surface[19].
Generally, the presence of fillers imparts greater mechanical strength to the polymeric film, reducing its flexibility.
An increasing loading, typically, produces a progressive permeation rate/performance enhancement, before achieving agglomeration or sticking phenomena, at high filler content, that are detrimental for selectivity target. The maximum loading depends on the filler type.
At the same size and content, synergistic effects are observed in the case of filler combinations versus single fillers in terms of better sorption properties and creation of selective gaps for CO2[20].
Filler pretreatment may be necessary in order to improve the dispersion within the polymer matrix, but it is not as stringent as in the case of glassy polymers. However, alternatively, appropriate physical linkages or specific chemical bonds facilitate the compatibility between the heterogeneous phases, improving the dispersion of the individual fillers and preventing particle agglomeration, but also modifying favorably the original polymer crystallinity or the free volume available for the gas molecules, with advantages in terms of final separation performance. Another aim of the filler functionalization is the introduction of chemical moieties for promoting the selective transport of CO2 with respect to other gases.
Specific additives, such as ionic liquids or PEGs, increase the mutual compatibility between the phases [21][22]. At the same time, they can make the matrix more flexible and convey the transport of some species when they bring appropriate functionalites[23].
Operation conditions affect the performance of the MMMs in a multiple way.
In a polymer matrix, as temperature increases, gas permeability rises, particularly for less permeable molecules. Indeed, gas diffusion is an activated process favored by temperature and it represent the main factor in which the permeability can be decoupled. The other term, the solubility, is negatively affected by the temperature, that reduces the sorption capacity of the species in the polymer matrix. As result of this combined effect, a decay in selectivity was observed.
The presence of the fillers can modify the behavior of the original matrix versus the gas permeation, also depending on additional transport mechanism occurrence. Therefore, unusual increases in CO2/N2 selectivity were observed at high temperature (e.g., 65°C[24]).
For what concerns an increase of the feed pressure, it causes a “densification” of the host matrix with a reduction of the permeation rate[13]. Similar trend was observed when carrier saturation occurs, combined to a separation factor decay [25][26]. Nevertheless, in the case of functionalized fillers, opposite behaviors can happen as effect of enhancement of gas sorption properties of specific polar gases (e.g. carbon dioxide) with respect to non-polar species (e.g. nitrogen or methane)[27][28].
Wet-state separations show a significantly different separation extent in comparison with the same operation carried out in dry-state. The humidity tends to swell the polymer causing lower resistance to the passage of gas molecules[29].
Commonly, the plasticization and swelling phenomena, occurring when CO2 permeates through the polymer matrix at moderate temperature and pressure conditions, are reduced as result of nanoparticles presence in MMMs, expanding their field of application.
References
- [P. Bernardo, G. Clarizia, A review of the recent progress in the development of Nanocomposites based on poly(ether-block-amide) copolymers as membranes for CO2 separation, Polymers 14/1 (2022) 10.]
- [R. Kardani, M. Asghari, T. Mohammadi, M. Afsari, Effects of nanofillers on the characteristics and performance of PEBA-based mixed matrix membranes, Reviews in Chemical Engineering 34/6 (2018) 797-836.]
- [W. Yave, A. Car, K.-V. Peinemann, Nanostructured membrane material designed for carbon dioxide separation, J. Membr. Sci. 350 (2010) 124–129.]
- [N. Azizi, S. Azizi, R. Homayoon, Experimental Study of CO2 and CH4 Permeability Values Through PebaxⓇ-1074/Silica Mixed Matrix Membranes, Silicon 11 (2019) 2045–2057., T. Barzegar, S. Hassanajili, Fabrication and characterization of dual layer PEBAX-SiO2/polyethersulfone nanocomposite membranes for separation of CO2/CH4 gases, Appl. Polym. Sci. (2021) e51624.]
- C. Song, M. Mujahid, R. Li, S. Ahmad, Q. Liu, B. Zhang, Y. Kitamura, Pebax/MWCNTs-NH2 mixed matrix membranes for enhanced CO2/N2 separation, Greenhouse Gas Sci. Technol. 10/2 (2020) 408–420.
- D. Wang, D. Yao, Y. Wang, F. Wang, Y. Xin, S. Song, Z. Zhang, F. Su, Y. Zheng, Carbon nanotubes and graphene oxide-based solvent-free hybrid nanofluids functionalized mixed-matrix membranes for efficient CO2/N2 separation, Separ. Purif. Tech. 221 (2019) 421–432
- . Wang, W. Zheng, X. Yang, M. Ning, X. Li, Y. Xi, X. Yan, X. Zhang, Y. Dai, H. Liu, G. He, Pebax-based mixed matrix membranes derived from microporous carbon nanospheres for permeable and selective CO2 separation, Separ. Purif. Tech. 274 (2021) 119015.
- MOF [J. Sánchez-Laínez, I. Gracia-Guillén, B. Zornoza, C. Téllez, J. Coronas, Thin supported MOF based mixed matrix membranes of Pebax® 1657 for biogas upgrade, New J. Chem. 43 (2019) 312–319
- W. Zheng, R. Ding, K. Yang, Y. Dai, X. Yan, G. He, ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs, Separ. Purif. Tech. 214 (2019) 111–119
- R. Ding, W. Zheng, K. Yang, Y. Dai, X. Ruan, X. Yan, G. He, Amino-functional ZIF-8 nanocrystals by microemulsion based mixed linker strategy and the enhanced CO2/N2 separation, Separ. Purif. Tech. 236 (2020) 116209
- Y. Zheng, Y. Wu, B. Zhang, Z. Wang, Preparation and characterization of CO2-selective Pebax/NaY mixed matrix membranes, J. Appl. Polym. Sci. 137 (2020) 48398
- B. Zhang, C. Yang, Y. Zheng, Y. Wu, C. Song, Q. Liu, Z. Wang, Modification of CO2-selective mixed matrix membranes by a binary composition of poly(ethylene glycol)/NaY zeolite, J. Membr. Sci. 627 (2021) 119239
- W. Zhu, F. Liu, M. Gou, R. Guo, X. Li, Mixed matrix membrane containing metal oxide nanosheets for efficient CO2 separation, Green Chem. Eng. 2/1 (2021) 132–143
- T.-C. Huang, Y.-C. Liu, G.-S. Lin, C.-H. Lin, W.-R. Liu, K.-L. Tung, Fabrication of pebax-1657-based mixed-matrix membranes incorporating N-doped few-layer graphene for carbon dioxide capture enhancement, J. Membr. Sci. 602 (2020) 117946
- R. Casadei, M. Giacinti Baschetti, M.J. Yoo, H.B. Park, L. Giorgini, Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture, Membranes 10(8) (2020) 188
- M.E. Kojabad, A. Babaluo, A. Tavakoli, A novel semi-mobile carrier facilitated transport membrane containing aniline/poly (ether-block-amide) for CO2/N2 separation: Molecular simulation and experimental study, Separ. Purif. Tech. 266 (2021) 118494
- P. Bernardo, G. Clarizia, Enhancing Gas Permeation Properties of Pebax® 1657 Membranes via Polysorbate Nonionic Surfactants Doping, Polymers 12(2) (2020) 253
- P. Bernardo, G. Clarizia, A review of the recent progress in the development of Nanocomposites based on poly(ether-block-amide) copolymers as membranes for CO2 separation, Polymers 14/1 (2022) 10.]
- W. Zhu, Y. Qin, Z. Wang, J. Zhang, R. Guo, X. Li, Incorporating the magnetic alignment of GO composites into Pebax matrix for gas separation, Journal of Energy Chemistry 31 (2019) 1–10
- F. Shi, J. Sun, J. Wang, M. Liu, S. Wang, X. Cao, Z. Yan, Y. Li, S.P. Nunes, Exploration of the synergy between 2D nanosheets and a non-2D filler in mixed matrix membranes for gas separation, Frontiers in Chemistry 8 (2020) 58
- G. Huang, A.P. Isfahani, A. Muchtar, K. Sakurai, B.B. Shrestha, D. Qin, D. Yamaguchi, E. Sivaniah, B. Ghalei, Pebax/ionic liquid modified graphene oxide mixed matrix membranes for enhanced CO2 capture, J. Membr. Sci. 565 (2018) 370–379
- C. Fallahi, S. Moradi, R. Behbahani, The Synthesis and Implementation of Pebax/PEG 400/NH2-MIL125 Nanocomposite Membranes to Separate CO2/CH4, Iranian Journal of Oil and Gas Science and Technology 8/2 - Serial Number 27 (2019) 107–127
- H. Zhao, Q. Xie, X. Ding, R. Cai, X. Tan, Y. Zhang, Advanced mixed matrix membranes of Pebax embedded with amino acid ionic liquids@PIM core-shell composite nanoparticles for CO2 separation, Separ. Purif. Tech. 263 (2021) 118350
- Zhao, H.; Xie, Q.; Ding, X.; Cai, R.; Tan, X.; Zhang, Y. Advanced mixed matrix membranes of Pebax embedded with amino acid ionic liquids@PIM core-shell composite nanoparticles for CO2 separation. Sep. Purif. Technol. 2021, 263, 118350
- S. Ding, X. Li, S. Ding, W. Zhang, R. Guo, J. Zhang, Ionic liquid-decorated nanocages for cooperative CO2 transport in mixed matrix membranes, Separ. Purif. Tech. 239 (2020) 116539
- Y. Wang, N. Zhang, H. Wu, Y. Ren, L. Yang, X. Wang, Y. Wu, Y. Liu, R. Zhao, Z. Jiang, Exfoliation-free layered double hydroxides laminates intercalated with amino acids for enhanced CO2 separation of mixed matrix membrane, J. Membr. Sci. 618 (2021) 118691
- M. Asghari, S. Saadatmandi, M. J. Parnian, Polypyrrole-aided surface decoration of graphene oxide nanosheets as fillers for poly(ether-b-amid) mixed matrix membranes to enhance CO2 capture, Int. J. Energy Res. 45/7 (2021) 10843–10857
- S.M.A. Ahmadi, T. Mohammadi, N. Azizi Superior Pebax-1657/amine-modified halloysite nanotubes mixed-matrix membranes to improve the CO2/CH4 separation efficiency, Journal of Applied Polymer Science 138/3115 (2021) Article number 50749
- Li, Y.; Li, X.; Wu, H.; Xin, Q.; Wang, S.; Liu, Y.; Tian, Z.; Zhou, T.; Jiang, Z.; Tian, H.; et al. Anionic surfactant-doped Pebax membrane with optimal free volume characteristics for efficient CO2 separation. J. Membr. Sci. 2015, 493, 460–469
