Diaper dermatitis is a common type of irritant contact dermatitis occurring in infants and toddlers. Its occurrence is triggered by an unfavorable environment under the diaper, damage to skin integrity by fecal enzyme degradation, overhydration and disruption of the lipid bilayer structure facilitating the entry of irritants and microorganisms. In diaper dermatitis development, the central proinflammatory cytokines are IL-1α, IL-8 and TNF-α. The initial release of IL-1α and TNF-α starts a further cascade of pro-inflammatory chemo- and cytokines, resulting in inflammation and erythema of the skin. A recently recognized factor in diaper dermatitis is the composition of the skin microbiome; common pathogenic strains Candida albicans and Staphylococcus aureus are associated with skin irritation. The resulting impaired microbiome composition produces a local inflammatory response and may thus worsen the initial dermatitis clinical presentation and subsequent healing.
1. Introduction
Diaper dermatitis (DD), also known as diaper/napkin/nappy rash, encompasses various infant dermatoses occurring in the perineal and perianal area [
1]. These eruptions may represent exacerbations of diffuse skin diseases, such as seborrheic dermatitis or atopic dermatitis (AD), or skin conditions that coincidently manifest in the diaper covered area [
2]. This entry uses the stricter definition, where DD is defined as a type of irritant contact dermatitis (ICD), occurring mostly in reaction to prolonged contact with urine, feces, or retained soaps and detergents (falling under the ICD10 diaper dermatitis L22 diagnosis), resulting in an acute inflammatory skin process [
3].
As one of the most prevalent skin conditions affecting babies, DD presents a problem to many parents after the birth of their child [
4]. It usually occurs in the first two years of life, especially between 9 and 12 months of age [
5,
6]. A recent study estimated the prevalence of DD in children under the age of 2 at around 36%, which decreased significantly with increasing age [
7]. DD typically affects the lower abdomen, thighs, and the entire area under the diaper, including intertriginous folds. DD initially presents with desiccation of the skin, followed by development of erythematous maceration and oedema [
3].
2. Microbiome Composition and Skin Immunology in Diaper Dermatitis
2.1. Early Fetal and Postnatal Microbiome
Adult skin is mainly colonized by four different phyla with quite stable dominant genera: Actinobacteria (most dominant reported genera:
Propionibacterium and
Corynebacterium), Firmicutes (most represented by
Lactobacillus,
Streptococcus and
Staphylococcus), Proteobacteria and Bacteroidetes, respectively [
13].
Dermal bacterial colonization is generally thought to begin at birth and continues forming throughout the first years of life and into adulthood [
14,
15]. The mode of fetal delivery is recognized as the major determinant of the newborn′s cutaneous microbiome composition [
15]. Recently, some authors proposed that maternal microbiota is selectively transported to the placenta to colonize the fetus already before birth. This was supported by reports of bacterial DNA in the placenta and amnion [
16]. The presence of oral and meconium microbiota has been reported at the time of Caesarean delivery, supposedly originating from the placenta [
17]. It appears this early lack of sterility may be protective. Non-sterile meconium-stained amniotic fluid is present in 5% to 20% of deliveries and was reported to reduce the risk of developing dermatitis and decreased skin-eruption-related hospitalizations throughout childhood and adolescence [
18].
Immediately after birth, infant skin bacterial communities have been reported to be similar across body sites [
19]. As soon as two days after delivery, bacterial communities begin to diverge into distinct functional communities at different skin sites, which are similar to those found in adults [
20]. The buttock skin bacteria soon form a separate microbiome environment, due to less likely competition from other skin sites to the diaper covered area and proximity to the gastrointestinal tract [
21]. Commonly isolated bacteria from the diaper area of neonates less than a week old are species of
Bifidobacteria and
Bacteroides, followed by
Enterobacteria, Eubacteria, Lactobacilli, amongst others [
12,
22].
Site-specific evolution of different bacterial communities appears to happen within the first three months of life [
21]. Six weeks after delivery, the skin microbiota of mother and infant are more similar than their microbiota at other body sites, like the gut and oropharynx, which diverge more rapidly [
23]. Over the first year of life the similarity between mother and infant microbiota further decreases as successful invasion of the environmental strains becomes more important [
24]. In time, the infant’s own gastrointestinal flora becomes an important contributor to the microbiota under the diaper, mainly colonized with
Clostridium species (spp.) and other gut-derived bacteria, and also significant amounts of
Bacteroides spp. [
12].
The gastrointestinal microbiota depends on various factors, especially the child’s diet: breastfed, bottle-fed or already weaned [
25,
26,
27]. Numerous indicators suggest important benefits of breastfeeding for child’s health during infancy and later in life [
28]. Because of the oligosaccharide content, breastfeeding stimulates intestinal proliferation of anaerobic microorganisms such as species of
Bifidobacteria (B. breves,
B. infantis,
B. pseudocatenulatum),
Lactobacilli and
Bacteroides. Bottle-feeding develops a mixed bacterial flora in the infant intestine, with a reduction of
Bifidobacteria spp. and a greater presence of
Bacteroides spp.,
Clostridia spp. and
Staphylococci spp.. When breast-feeding is supplemented with bottle-feeding, the profile of intestinal microflora is similar to formula-fed infants [
25,
26,
27]. Weaning is the main milestone when the intestinal flora becomes similar to that of an adult, with an increase in
Bacteroides spp. and anaerobic gram-positive bacteria, such as
Peptococci spp.,
Peptostreptococci spp.,
Veillonella spp. and
Staphylococci spp. [
27].
Early in life, the microbiome is highly plastic. It undergoes dynamic changes until the age of 3 to 4 years, when it stabilizes and becomes more adult-like [
29]. Because of the shifting nature of microbiomes in preschool children, we believe probiotics might be especially beneficial in treating babies with DD.
2.2. The Microbiome in Diaper Dermatitis
The buttock area is unique in its microbiota composition with an early colonization with aerobic bacteria (
Staphylococcus spp., Streptococcus spp. and
Enterococcus spp.) and other transitional taxa such as
Prevotella spp., Veillonella spp. and
Clostridion spp.. In children with DD, the affected skin is highly susceptible to microbial infections, in particular to intestinal microbial residues [
30]. In addition, both secondary bacterial and
Candida infections can complicate dermatitis [
3]. In DD specifically, colonization with
Finegoldia [
31] has been proposed as an important contributing factor because of its higher-than-expected abundance on the buttock skin area, but the mechanism is still unclear [
21]. An increased presence of
Enterococci, which are normal skin and gut bacteria with a pathogenic potential, has also been shown [
32].
These results show that the composition of the normal skin microbiota in diaper dermatitis is altered. Bacterial diversity in DD patients is higher compared with healthy controls [
30]. This contrasts the generally accepted notion of a beneficial effect of greater bacterial diversity, as seen in AD [
13].
When it comes to skin flora, the DD affected area showed a paucity of beneficial strains like
Staphylococcus epidermidis,
Bifidobacterium longum,
Clostridium butyricum and
Lactobacillus ruminis [
30]. Although the colonization with
Staphylococcus spp. decreases with increasing DD severity, the more severe DD the predominant
Staphylococcus spp. is
Staphylococcus aureus, potentially implicating
S. aureus as a DD etiological agent. In contrast, the demonstrated community percentages of fecal coliforms increase with DD severity [
33]. The most often isolated pathogenic strains are
Candida albicans and
S. aureus. It is reasonable to believe that they play a predominant role in DD [
12], but further research on the impact of microbiome composition on DD is certainly needed.
2.3. Inflammation in Diaper Dermatitis
Skin inflammation is generally triggered by external factors such as allergen intake, contact with microbes or with irritants, UV radiation and by other, less well-defined stimuli [
34]. In DD skin irritation is affected by adverse fecal enzymes, friction and subsequent skin maceration, high pH, the presence of urine and prolonged skin contact with feces [
6]. Inadequate skin care, certain microbial invasion, antibiotic use and a lack of individual nutrients also play a role (
Figure 1) [
3]. The resulting inflammatory process involves a complex interplay of events between skin cells, immune cells, inflammatory cytokines, and chemokines [
35].
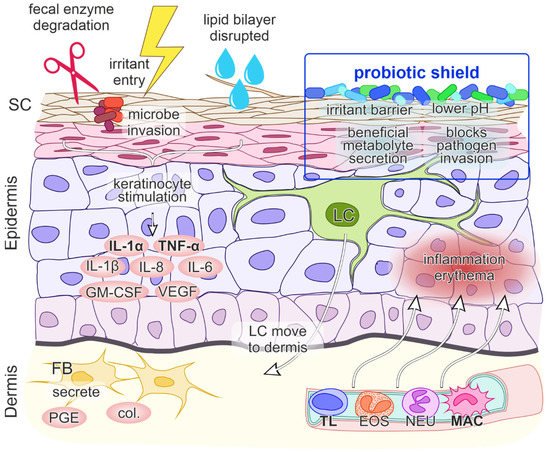
Figure 1. A model of contact irritant diaper dermatitis. The first step involves the penetration of irritants through the skin which stimulates keratinocytes (KC) to release proinflammatory mediators interleukin 1 alpha (IL-1α) and tumor necrosis factor alpha (TNF-α). The entry of irritants and microorganisms is facilitated by damage of stratum corneum (SC) integrity by fecal enzyme degradation, overhydration and disruption of the lipid bilayer structure. The initial release of IL-1α and TNF-α promotes further production of cytokines and chemokines IL-1β, granulocyte macrophage-colony stimulating factor (GM-CSF), IL-6, IL-8, vascular endothelial growth factor (VEGF), migration of Langerhans cells (LC) to the dermis, production of collagenases (col.) and prostaglandin E (PGE) by fibroblasts (FB), vasodilatation of the blood vessels, upregulation of adhesion molecules on endothelial cells and the transmigration of inflammatory cells (TL—T lymphocyte, EOS—eosinophil, NEU—neutrophil, MAC—macrophage) to the epidermis. The net effect is inflammation and erythema of the skin. Probiotics present a protective shield against irritants, maintain a lower pH, secrete beneficial metabolites and block pathogen invasion.
2.4. Role of Skin pH
Across body sites pH values vary from pH 4.0 to 7.0. Around half reports describe pH values below 5.0, which contrasts with the general assumption of a healthy skin pH being between 5.0 to 6.0. Low pH values below 5.0 are associated with favorable barrier function, moisturization and scaling. A proposed mechanism for low pH protective effect is that an acidic skin pH between 4.0–4.5 keeps the resident bacterial flora attached to the skin, whereas an alkaline pH between 8.0–9.0 promotes dispersal from the skin [
36]. Maintenance of the acidic layer of the epidermis is also of major importance as it maintains a protective system of the skin and creates an unfavorable environment for colonization by pathogenic microorganisms [
37]. Ammonia-induced alkalinization activates fecal enzymes such as lipase and trypsin, leading to irritation and disruption of the skin barrier. However, the skin in some cases of infants has already been reported to have an elevated baseline pH of 6.6 [
38], suggesting a possible predisposition for DD development.
2.5. Inflammatory Signaling
At a molecular level, when DD results from a contact etiology, irritant penetration through the skin induces endogenous “danger” signals, which cause direct damage to keratinocytes and the release of cytokines and chemokines IL-1α, IL-1β and TNF-α. These cytokines unsurprisingly play a central role in DD immune system activation, as they can trigger inflammation on their own. Keratinocytes additionally secrete the granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6 and IL-8, where IL-8 is a potent chemokine for lymphocytes and IL-6 influences the process of maturation of keratinocytes. The initial signal release is followed by the migration of Langerhans cells to the dermis, the production of collagenases and prostaglandin E by fibroblasts and the upregulation of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on keratinocytes, fibroblasts and endothelial cells. Subsequently, blood vessels dilate and passage of inflammatory cells into the epidermis ensues, leading to inflammation and erythema (
Figure 1) [
35,
46].
Less well characterized cytokines that are involved in ICD are CCL (chemokine (C-C motif) ligand) 20, CCL27, IL-10, IL-12 and IL-18 [
35]. The cytokines that are primarily upregulated following irritant exposure are IL-1α and TNF-α [
35,
43,
46]. Although the precise cytokines/chemokines activation cascade in DD is still unclear, IL-8 has also been specifically implicated as a strong contributor in DD [
11,
42].
2.6. Probiotics as a “Protective Shield” against Skin Inflammation
Probiotics exert health effects on the skin directly through cutaneous formulations or indirectly through dietary supplementary formulations and intestinal microflora improvement [
52]. Certain probiotics can modulate the cutaneous microflora, the lipid barrier, and the skin immune system, leading to the maintenance of skin homeostasis [
53].
Beneficial dermal effects of probiotics have been shown via oral consummation through acting on the intestine with changes in systemic immune responses and thus immunomodulation of the skin, inhibition of allergen-induced tumors via changes in systemic immune responses and inhibition of harmful intestinal microflora. Probiotics can also act as antioxidant agents. Probiotics may be applied directly on the skin which then compete with harmful skin microflora, secrete useful metabolites, reduce pH and act as a barrier to harmful foreign environmental factors that are in contact with the skin (
Figure 1) [
52].
As the interest in the use of probiotics in DD is very recent, there is currently only very limited data available on their use as a DD prevention or treatment option. An older preliminary report from 1998 describes a trial of infant formula supplemented with
Bifidobacterium lactis and
Streptococcus thermophilus, which showed a small decrease in DD incidence compared to non-supplemented formula during the observation period [
54]. Examining probiotics as a DD treatment, a single 2021 article favourably reports on a market research study that followed an at-home use of oral activated
Bifidobacterium infantis EVC001 in infants with DD [
55]. Although the consumer feedback was very positive, this type of research is methodologically limited and potentially biased. Consequently, more rigorous scientific research on use of probiotics in DD is desired.
3. Conclusions and Outlook
Although the immediate cause of DD is contact with environmental irritants, the individual predispositions to its development and severity are less clear. The microbiome composition seems to be an important factor. Evidence for microbiota modulation with probiotics in children exists mainly for atopic dermatitis, where some studies reported positive results [
27].
The exact pathophysiological mechanism of probiotic immune system modulatory effects and counteraction of inflammation processes in DD is yet to be understood. IL-1α, TNFα and IL-8 seem to play a central role in driving DD inflammation, but exact involvement of other cytokines and chemokines is still to be uncovered. Furthermore, the interplay of inflammatory pathway activation and the microbiome require further investigation.
This entry is adapted from the peer-reviewed paper 10.3390/children9010112