Bilberry (Vaccinium myrtillus L.) is a natural resource and a useful wild berry in Europe. Various parts of the plant contain many benefits for human health. The adaptation and secondary metabolism of V. myrtillus plants can be synergistically affected by a community of microbial endophytes.
1. Introduction
Nutritional composition and antioxidant activity due to the abundance of phenolic compounds in leaves extracts are beneficial to human health [14,15]. The growing area of V. myrtillus is native to Europe, but this species is also found in temperate and sub-Arctic regions around the world. Bilberries are widely abundant and easily found in the forests of Lithuania, Latvia, Finland, and Norway. Well-drained, moist, acidic soils are best for this species, but it can also grow in very acidic soils (pH 4.5–6). Thus, the adaptation and secondary metabolism of V. myrtillus plants can be synergistically affected by microbial endophytes, the benefits and potential of which are, in many cases, unknown in wild forest plants. In addition, some endophytes have shown a good ability to colonize host plant tissues; therefore, bacteria have a beneficial effect on plant growth by providing plants with the necessary nutrients or bioactive compounds [16,17,18,19]. Many beneficial microorganisms from different plant species and environments have recently been identified that can act as sources of new bioactive compounds and can therefore be used in the medical, agricultural or food industries. Numerous microbiological and ecological studies have shown that plant endophytes and their products may be promising candidates as a biological control measure.
2. Biodiversity of Endophytic Bacteria in Bilberry Leaves
A total of 25 genetically distinct endophytic bacteria were isolated from the leaves of V. myrtillus according to the sequence data of the 16S rRNA gene (Table 1). BLAST and phylogenetic analysis of 16S rRNA gene sequences revealed that the endophytic bacterial isolates were 99.58–100% similar to the sequences available in the NCBI GenBank. The 16S rDNA nucleotide sequences were submitted to GenBank and assigned accession numbers MZ469297 to MZ469321. The genetic identity among the sequences was found to be 100% in 18 bacteria strains. The 16S rRNA gene sequences demonstrated that strains Bil-LT1_1, Bil-LT1_2, Bil-LT4_7, Bil-LV3_1, and Bil-FIN2_3 were identical to several Bacillus spp. species from NCBI; however, due to genetic similarity within the Bacillus spp. species, precise identification of the isolates should be performed for further studies. The same situation was observed for strains Bil-LT4_8 and Bil-NOR3_14, which were related to Micrococcus sp., and strains Bil-Bil-2_5, Bil-Bil-2_6, and Bil-NOR3_11, which were related to Staphylococcus sp. One strain, Bil-FIN2_7, was related to Lysinibacillus spp. Three strains (Bil-LV3_4, LV3_6, and NOR3_18) with minor nucleotide polymorphisms were closely related to Paenibacillus spp. Strains of endophytic bacteria, such as Rothia amarae (Bil-LT4_1), Paenibacillus tundrae (Bil-LV3_3), Kocuria kristinae (Bil-FIN2_9, Bil-FIN2_13), Weissella hellenica (Bil-FIN2_10), Micrococcus terreus (Bil-FIN2_12), Corynebacterium freneyi (Bil-NOR3_13), Pseudomonas monteilii (Bil-NOR3_15), Sporosarcina aquimarina (Bil-NOR3_16), and Paenibacillus xylanexedens (Bil-NOR3_17), were identified in bilberry leaves.
Table 1. Comparative matches for the closest phylogenetic genotypes (according NCBI records) obtained for the culturable bacteria isolates based on profile of 16S rRNA gene.
| Isolate |
Accession Number in NCBI |
Identity Accessions, According NCBI |
Sequence Length, bp (Identity, %) |
| Bil-LT1_1 |
MZ469297 |
Bacillus halotolerans MK517597.1
B. mojavensis MF040286.1
B. velezensis MT634548.1
B. axarquiensis GU568194.1
B. subtilis AB526464.1 |
1437 (100) |
| Bil-LT1_2 |
MZ469298 |
Bacillus simplex LK391525.1
Peribacillus butanolivorans CP050509.1 |
1431 (100) |
| Bil-LT4_1 |
MZ469299 |
Rothia amarae MG905369.1 |
1400 (99.79) |
| Bil-LT4_3 |
MZ469300 |
Bacterium strain MTL8-4 MH151301.1 |
1439 (99.58) |
| Bil-LT4_7 |
MZ469301 |
Bacillus zhangzhouensis MN826587.1
B. pumilus CP054310.1
B. safensis KJ542766.1
B. stratosphericus KY203662.1 |
1420 (100) |
| Bil-LT4_8 |
MZ469302 |
Micrococcus sp. MG132043.1
M. luteus AJ409096.1 |
1398 (100) |
| Bil-LV3_1 |
MZ469303 |
Bacillus sp. strain MK736127.1
B. aryabhattai MN515130.1
B. megaterium MF988696.1 |
1427 (100) |
| Bil-LV3_3 |
MZ469304 |
Paenibacillus tundrae HF545335.1 |
1431 (100) |
| Bil-LV3_4 |
MZ469305 |
Paenibacillus sp. MK290403.1 |
1435 (99.65) |
| Bil-LV3_6 |
MZ469306 |
Paenibacillus sp. MG758020.1 |
1450 (99.86) |
| Bil-FIN2_3 |
MZ469307 |
Bacillus cereus MN068934.1
B. thuringiensis CP050183.1 |
1439 (100) |
| Bil-FIN-2_5 |
MZ469308 |
Staphylococcus warneri CP038242.1
S. pasteuri MW433878.1 |
1437 (100) |
| Bil-FIN2_6 |
MZ469309 |
Staphylococcus warneri CP038242.1
S. pasteuri MW433878.1 |
1437 (100) |
| Bil-FIN2_7 |
MZ469310 |
Lysinibacillus macrolides MH542661.1
L. xylanilyticus KP644237.1
L. fusiformis FJ641020.1 |
1427 (100) |
| Bil-FIN2_9 |
MZ469311 |
Kocuria kristinae KX055834.1 |
1384 (100) |
| Bil-FIN2_10 |
MZ469312 |
Weissella hellenica CP042399.1 |
1447 (100) |
| Bil-FIN2_12 |
MZ469313 |
Micrococcus terreus KJ781899.1 |
1385 (100) |
| Bil-FIN2_13 |
MZ469314 |
Kocuria kristinae KX055834.1 |
1384 (100) |
| Bil-NOR3_11 |
MZ469315 |
Staphylococcus sp. KM253075.1 |
1431 (100) |
| Bil-NOR3_13 |
MZ469316 |
Corynebacterium freneyi EF462412.1 |
1393 (99.86) |
| Bil-NOR3_14 |
MZ469317 |
Micrococcus sp. KX350143.1
M. luteus MN826463.1 |
1385 (100) |
| Bil-NOR3_15 |
MZ469318 |
Pseudomonas monteilii CP013997.1 |
1422 (100) |
| Bil-NOR3_16 |
MZ469319 |
Sporosarcina aquimarina MK726086.1 |
1433 (100) |
| Bil-NOR3_17 |
MZ469320 |
Paenibacillus xylanexedens CP018620.1 |
1436 (99.79) |
| Bil-NOR3_18 |
MZ469321 |
Paenibacillus sp. KR055031.1 |
1427 (99.86) |
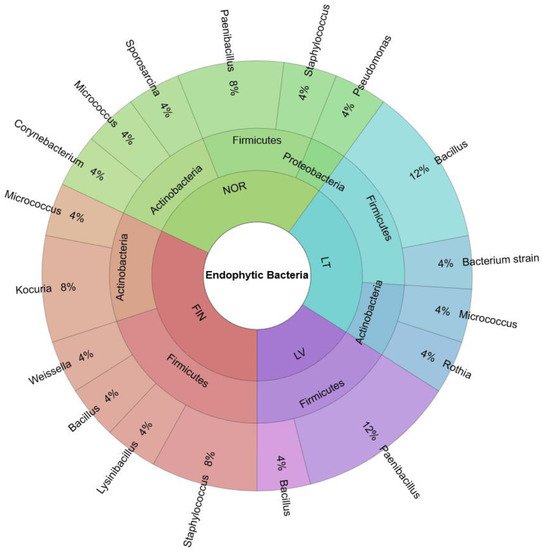
The biodiversity of endophytic bacteria in bilberry leaves from the countries of the Baltic-Nordic region (Lithuania, Latvia, Finland, and Norway) was investigated and is shown in Figure 1. Endophytic bacteria isolated from bilberry leaves belonged to the phyla Firmicutes 64%, Actinobacteria 32%, and Proteobacteria 4%. Bacteria of the families Bacillaceae, Paenibacillaceae, and Micrococcaceae were the most frequently isolated and identified in blueberry leaves. Staphylococcaceae, Lactobacillaceae, Pseudomonaceae, Corynebacteriaceae, and Planococcaceae strains were identified in one or more cases.
Figure 1. Biodiversity of endophytic bacteria in bilberry leaves of the Baltic-Nordic region’s forests—Lithuania (LT), Latvia (LV), Finland (FIN) and Norway (NOR).
Endophytic bacteria isolated from Norwegian bilberry leaves belonged to Firmicutes 1%, Actinobacteria 12%, and Proteobacteria 4% phyla. Meanwhile, bacteria in Finnish and Lithuanian leaf samples belonged to Firmicutes (20% and 16%, respectively) and Actinobacteria (12% and 8%, respectively). Only Firmicutes phylum bacteria were isolated from the Latvian samples. Bacteria of the Bacillaceae family were isolated and identified in the bilberry leaves of Lithuania, Latvia, and Finland, but were not found in plant samples from Norway. Bacteria belonging to six different families isolated from leaves samples were collected from the northern countries—Finland and Norway (Figure 2).
3. Enzymatic-Genetic Features of Endophytic Bacteria in Bilberry Leaves
Enzymatic activity was tested in all bacterial endophytes isolated from bilberry leaves (Table 2). The results showed that amylase was detected in 44% of the tested isolates, proteases in 56%, and catalases in 88%. The study showed that seven bacterial endophytes isolated from bilberry leaves were able to produce amylase, protease, and catalase. All bacterial endophytes tested had at least one enzymatic activity, except for one bacterial strain, Weissella hellenica Bil-FIN2_10, which lacked amylase, protease, and catalase activity.
Table 2. Enzymatic activity and the presence of the genes acdS (1-aminocyclopropane-1-carboxylate deaminase (ACCD)) and AcPh (acid phosphatase) in endophytic bacteria isolated from bilberry leaves of the Baltic-Nordic region.
Endophytic Bacteria Strains
in Different Geographic Locations |
Amylolytic Activity, mm |
Proteolytic Activity, mm |
Catalase Reaction |
Gene acdS |
Gene AcPho |
| Bacillus sp. Bil-LT1_1 |
11.9 ± 0.1 |
10.0 ± 0.1 |
+ |
+ |
- |
| Bacillus sp. Bil-LT1_2 |
|
|
+ |
- |
- |
| Rothia amarae Bil-LT4_1 |
12.0 ± 0.2 |
11.8 ± 0.2 |
+ |
- |
- |
| Bacterium strain Bil-LT4_3 |
|
9.8 ± 0.1 |
+ |
- |
- |
| acillus sp. Bil-LT4_7 |
9.0 ± 0.2 |
10.2 ± 01 |
+ |
- |
- |
| Micrococcus sp. Bil-LT4_8 |
|
9.3 ± 0.1 |
+ |
- |
- |
| Bacillus sp. Bil-LV3_1 |
12.5 ± 0.1 |
14.2 ± 0.2 |
+ |
- |
- |
| Paenibacillus tundrae Bil-LV3_3 |
10.1 ± 0.2 |
11.9 ± 0.2 |
+ |
- |
- |
| Paenibacillus sp. Bil-LV3_4 |
12.1 ± 0.1 |
10.2 ± 0.1 |
+ |
- |
- |
| Paenibacillus sp. Bil-LV3_6 |
|
10.2 ± 0.1 |
- |
- |
- |
| Bacillus sp. Bil-FIN2_3 |
12.3 ± 0.2 |
14.3 ± 0.3 |
- |
+ |
+ |
| Staphylococcus sp. Bil-FIN2_5 |
|
|
+ |
- |
- |
| Staphylococcus sp. Bil-FIN2_6 |
11.9 ± 0.2 |
|
+ |
- |
- |
| Lysinibacillus sp. Bil-FIN2_7 |
|
|
+ |
- |
- |
| Kocuria kristinae Bil-FIN2_9 |
|
|
+ |
+ |
- |
| Weissella hellenica Bil-FIN2_10 |
|
|
- |
- |
- |
| Micrococcus terreus Bil-FIN2_12 |
|
9.5 ± 0.1 |
+ |
- |
+ |
| Kocuria kristinae Bil-FIN2_13 |
|
8.8 ± 0.1 |
+ |
- |
+ |
| Paenibacillus sp. Bil-NOR3_17 |
|
10.1 ± 0.2 |
+ |
- |
- |
| Paenibacillus sp. Bil-NOR3_18 |
|
|
+ |
- |
- |
| Corynebacterium sp. Bil-NOR3_13 |
12.2 ± 0.1 |
|
+ |
- |
- |
| Micrococcus sp. Bil-NOR3_14 |
10.3 ± 0.2 |
14.5 ± 0.2 |
+ |
- |
- |
| Staphylococcus sp. Bil-NOR3_11 |
10.1 ± 0.1 |
|
+ |
- |
- |
| Pseudomonas monteilii Bil-NOR3_15 |
|
|
+ |
- |
- |
| Sporosarcina aquimarina Bil-NOR3_16 |
|
|
+ |
- |
- |
Two genes contributing to PGP traits were screened in the endophytic bacterial community. The genes responsible for the synthesis of ACC deaminases (acdS) and/or acid phosphatase (AcPho) were found in five (20%) isolates of endophytic bacteria (Table 2) belonging to the Mocrococcaceae and Bacillaceae families. Four isolates of endophytic bacteria with genes responsible for overcoming salinity stress and helping absorb insoluble phosphorus from forest soils were found in Finnish leaf samples, and one was found in samples collected in Lithuania. One isolate, Bacillus sp. Bil-FIN2_3, yielded specific PGR fragments of the expected size for both genes. Isolates of Bacillus sp. Bil-LT1_1 and Kocuria kristinae Bil-FIN2_9 produced specific PCR products of the expected size (~850 bp) of the acdS gene. Two bacterial strains from Finland, Micrococcus terreus Bil-FIN2_12 and Kocuria kristinae Bil-FIN2_13, gave an amplified fragment of the expected size (~734 bp) for the AcPho gene, indicating the potential ability of these isolates to produce acid phosphatase.
4. Summary
A community of 25 microorganisms was found in the leaves of bilberry (Vaccinium myrtillus L.). Isolates with proteolytic and amylases activity indicated the possible expression of these enzymes and their potential role in the degradation of starch and protein organic matter in the ecosystem. Ninety-six percent of endophytic bacteria strains of V. myrtillus L. had positive enzymatic activity and 20% had functional plant growth-promoting traits. The accumulation of this new collection of microorganisms and the primary genetic-enzymatic analysis opens opportunities for the study of some isolates against pathogenic organisms and for reducing the effects of salinity stress on other plants.
This entry is adapted from the peer-reviewed paper 10.3390/f12121647