Exposure to Endocrine Disrupting Chemicals (EDC) has been linked with several adverse outcomes. Transcriptome-wide analyses using RNAseq provide snapshots of cellular, tissue, and whole organism transcriptomes under normal physiological and EDC perturbed conditions. A global view of gene expression provides highly valuable information as it uncovers gene families or more specifically, pathways that are affected by EDC exposures, but also reveals those that are unaffected. Hypotheses about genes with unknown functions can also be formed by comparison of their expression levels with genes of known function.
- Endocrine Disrupting Chemicals (EDC)
- DNA microarrays
- RNA sequencing
- Adverse Outcome Pathway (AOP)
- Molecular Initiating Event (MIE)
- Key Events (KE)
- risk assessment
1. Introduction
2. Genomics and Bioinformatics Approaches in Endocrine Disruptor Research
2.1. RNA Sequencing
2.2. RNAseq for the Study of Endocrine Disruption
3. The Adverse Outcomes Framework
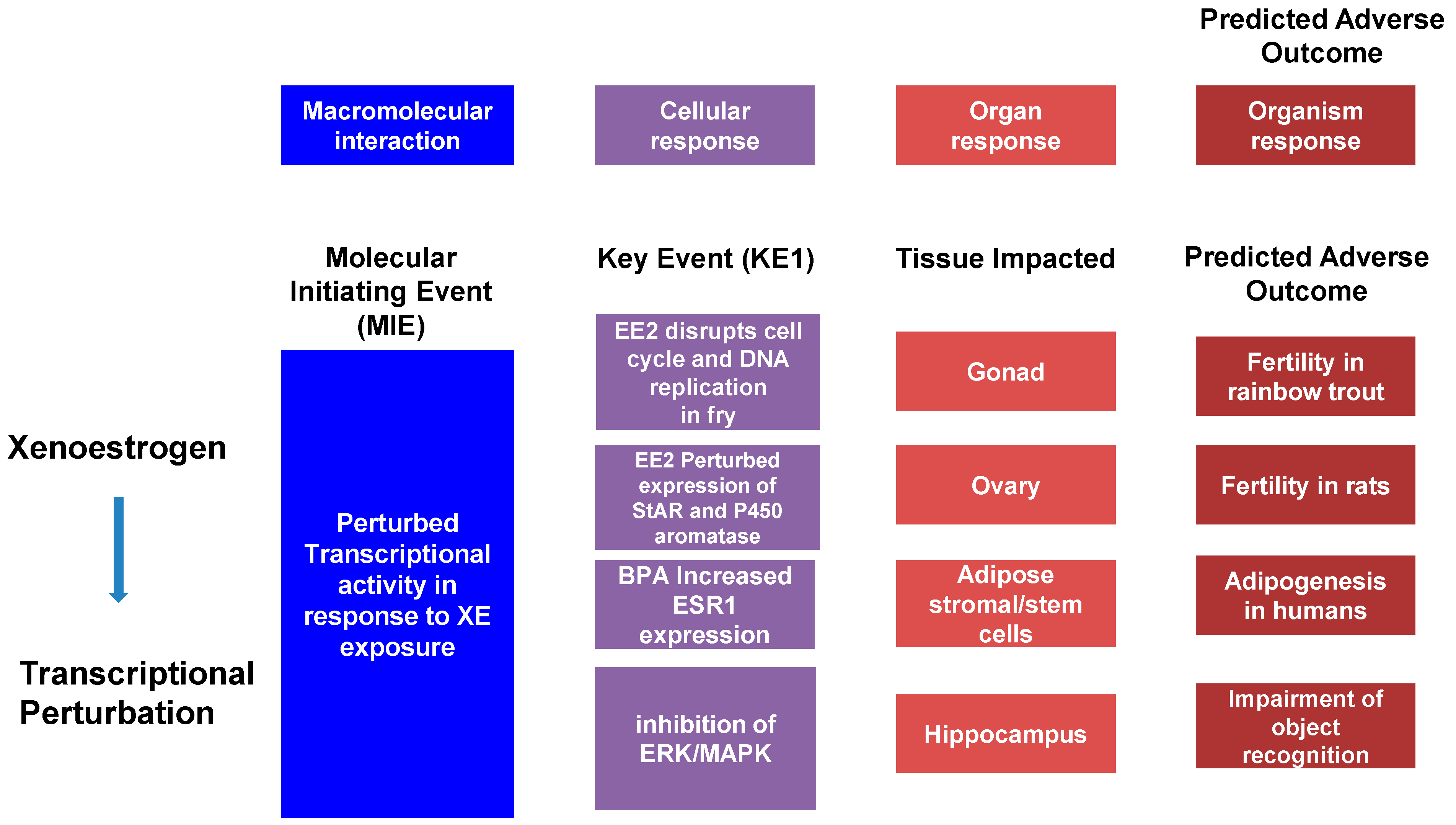
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19010574
References
- Chen, G.; White, P.A. The Mutagenic Hazards of Aquatic Sediments: A Review. Mutat. Res. 2004, 567, 151–225.
- Baker, M.E.; Ruggeri, B.; Sprague, L.J.; Eckhardt-Ludka, C.; Lapira, J.; Wick, I.; Soverchia, L.; Ubaldi, M.; Polzonetti-Magni, A.M.; Vidal-Dorsch, D.; et al. Analysis of Endocrine Disruption in Southern California Coastal Fish Using an Aquatic Multispecies Microarray. Environ. Health Perspect. 2009, 117, 223–230.
- Grün, F.; Watanabe, H.; Zamanian, Z.; Maeda, L.; Arima, K.; Cubacha, R.; Gardiner, D.M.; Kanno, J.; Iguchi, T.; Blumberg, B. Endocrine-Disrupting Organotin Compounds Are Potent Inducers of Adipogenesis in Vertebrates. Mol. Endocrinol. 2006, 20, 2141–2155.
- Lam, S.H.; Mathavan, S.; Tong, Y.; Li, H.; Karuturi, R.K.M.; Wu, Y.; Vega, V.B.; Liu, E.T.; Gong, Z. Zebrafish Whole-Adult-Organism Chemogenomics for Large-Scale Predictive and Discovery Chemical Biology. PLoS Genet. 2008, 4, e1000121.
- Lange, A.; Paull, G.C.; Coe, T.S.; Katsu, Y.; Urushitani, H.; Iguchi, T.; Tyler, C.R. Sexual Reprogramming and Estrogenic Sensitization in Wild Fish Exposed to Ethinylestradiol. Environ. Sci. Technol. 2009, 43, 1219–1225.
- Soto, A.M.; Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C. Does Breast Cancer Start in the Womb? Basic Clin. Pharmacol. Toxicol. 2008, 102, 125–133.
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95.
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57.
- Vajda, A.M.; Barber, L.B.; Gray, J.L.; Lopez, E.M.; Woodling, J.D.; Norris, D.O. Reproductive Disruption in Fish Downstream from an Estrogenic Wastewater Effluent. Environ. Sci. Technol. 2008, 42, 3407–3414.
- Toporova, L.; Balaguer, P. Nuclear Receptors Are the Major Targets of Endocrine Disrupting Chemicals. Mol. Cell. Endocrinol. 2020, 502, 110665.
- Litwa, E.; Rzemieniec, J.; Wnuk, A.; Lason, W.; Krzeptowski, W.; Kajta, M. RXRalpha, PXR and CAR Xenobiotic Receptors Mediate the Apoptotic and Neurotoxic Actions of Nonylphenol in Mouse Hippocampal Cells. J. Steroid Biochem. Mol. Biol. 2016, 156, 43–52.
- Huang, W.; Wu, Q.; Xu, F.; Li, L.; Li, J.; Que, H.; Zhang, G. Functional Characterization of Retinoid X Receptor with an Emphasis on the Mediation of Organotin Poisoning in the Pacific Oyster (Crassostrea gigas). Gene 2020, 753, 144780.
- Inderbinen, S.G.; Engeli, R.T.; Rohrer, S.R.; Di Renzo, E.; Aengenheister, L.; Buerki-Thurnherr, T.; Odermatt, A. Tributyltin and Triphenyltin Induce 11β-Hydroxysteroid Dehydrogenase 2 Expression and Activity through Activation of Retinoid X Receptor α. Toxicol. Lett. 2020, 322, 39–49.
- Milton, F.A.; Lacerda, M.G.; Sinoti, S.B.P.; Mesquita, P.G.; Prakasan, D.; Coelho, M.S.; de Lima, C.L.; Martini, A.G.; Pazzine, G.T.; Borin, M.d.F.; et al. Dibutyltin Compounds Effects on PPARγ/RXRα Activity, Adipogenesis, and Inflammation in Mammalians Cells. Front. Pharm. 2017, 8, 507.
- Lopes, C.; Madureira, T.V.; Ferreira, N.; Pinheiro, I.; Castro, L.F.; Rocha, E. Peroxisome Proliferator-Activated Receptor Gamma (PPARgamma) in Brown Trout: Interference of Estrogenic and Androgenic Inputs in Primary Hepatocytes. Environ. Toxicol. Pharm. 2016, 46, 328–336.
- Fang, H.; Fang, W.; Cao, H.; Luo, S.; Cong, J.; Liu, S.; Pan, F.; Jia, X. Di-(2-Ethylhexyl)-Phthalate Induces Apoptosis via the PPARγ/PTEN/AKT Pathway in Differentiated Human Embryonic Stem Cells. Food Chem. Toxicol. 2019, 131, 110552.
- Shoaito, H.; Petit, J.; Chissey, A.; Auzeil, N.; Guibourdenche, J.; Gil, S.; Laprévote, O.; Fournier, T.; Degrelle, S.A. The Role of Peroxisome Proliferator–Activated Receptor Gamma (PPARγ) in Mono(2-Ethylhexyl) Phthalate (MEHP)-Mediated Cytotrophoblast Differentiation. Environ. Health Perspect. 2019, 127, 27003.
- Parillo, F.; Maranesi, M.; Brecchia, G.; Gobbetti, A.; Boiti, C.; Zerani, M. In Vivo Chronic and in Vitro Acute Effects of Di(2-Ethylhexyl) Phthalate on Pseudopregnant Rabbit Corpora Lutea: Possible Involvement of Peroxisome Proliferator-Activated Receptor Gamma. Biol. Reprod. 2014, 90, 41.
- Kim, Y.S.; Hwang, K.A.; Hyun, S.H.; Nam, K.H.; Lee, C.K.; Choi, K.C. Bisphenol A and Nonylphenol Have the Potential to Stimulate the Migration of Ovarian Cancer Cells by Inducing Epithelial-Mesenchymal Transition via an Estrogen Receptor Dependent Pathway. Chem. Res. Toxicol. 2015, 28, 662–671.
- Kitraki, E.; Nalvarte, I.; Alavian-Ghavanini, A.; Ruegg, J. Developmental Exposure to Bisphenol A Alters Expression and DNA Methylation of Fkbp5, an Important Regulator of the Stress Response. Mol. Cell. Endocrinol. 2015, 417, 191–199.
- Jiang, J.; Ma, L.; Yuan, L.; Wang, X.; Zhang, W. Study on Developmental Abnormalities in Hypospadiac Male Rats Induced by Maternal Exposure to Di-n-Butyl Phthalate (DBP). Toxicology 2007, 232, 286–293.
- Liu, K.; Lehmann, K.P.; Sar, M.; Young, S.S.; Gaido, K.W. Gene Expression Profiling Following in Utero Exposure to Phthalate Esters Reveals New Gene Targets in the Etiology of Testicular Dysgenesis. Biol. Reprod. 2005, 73, 180–192.
- Fudvoye, J.; Lopez-Rodriguez, D.; Franssen, D.; Parent, A.-S. Endocrine Disrupters and Possible Contribution to Pubertal Changes. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101300.
- Saaristo, M.; Wong, B.B.; Mincarelli, L.; Craig, A.; Johnstone, C.P.; Allinson, M.; Lindstrom, K.; Craft, J.A. Characterisation of the Transcriptome of Male and Female Wild-Type Guppy Brains with RNA-Seq and Consequences of Exposure to the Pharmaceutical Pollutant, 17alpha-Ethinyl Estradiol. Aquat. Toxicol. 2017, 186, 28–39.
- Komada, M.; Gendai, Y.; Kagawa, N.; Nagao, T. Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Impairs Development of the Mouse Neocortex. Toxicol. Lett. 2016, 259, 69–79.
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics 2011, 128, 873–882.
- Wang, C.; Li, Z.; Han, H.; Luo, G.; Zhou, B.; Wang, S.; Wang, J. Impairment of Object Recognition Memory by Maternal Bisphenol A Exposure Is Associated with Inhibition of Akt and ERK/CREB/BDNF Pathway in the Male Offspring Hippocampus. Toxicology 2016, 341–343, 56–64.
- Chen, F.; Zhou, L.; Bai, Y.; Zhou, R.; Chen, L. Hypothalamic-Pituitary-Adrenal Axis Hyperactivity Accounts for Anxiety- and Depression-like Behaviors in Rats Perinatally Exposed to Bisphenol A. J. Biomed. Res. 2015, 29, 250–258.
- Yu, C.J.; Du, J.C.; Chiou, H.C.; Yang, S.H.; Liao, K.W.; Yang, W.; Chung, M.Y.; Chien, L.C.; Hwang, B.; Chen, M.L. Attention Deficit/Hyperactivity Disorder and Urinary Nonylphenol Levels: A Case-Control Study in Taiwanese Children. PLoS ONE 2016, 11, e0149558.
- Morii, K.; Nishisaka, M.; Nakamura, S.; Oda, T.; Aoyama, Y.; Yamamoto, T.; Kishida, H.; Okushin, H.; Uesaka, K. A Case of Synthetic Oestrogen-Induced Autoimmune Hepatitis with Microvesicular Steatosis. J. Clin. Pharm. 2014, 39, 573–576.
- Tao, J.; Sun, L.X.; Le, X.C. Study of the Effects of Bisphenol A Using Human Fetal Lung Fibroblasts. J. Environ. Sci. 2016, 48, 6–10.
- Koike, E.; Yasuda, Y.; Shiota, M.; Shimaoka, M.; Tsuritani, M.; Konishi, H.; Yamasaki, H.; Okumoto, K.; Hoshiai, H. Exposure to Ethinyl Estradiol Prenatally and/or after Sexual Maturity Induces Endometriotic and Precancerous Lesions in Uteri and Ovaries of Mice. Congenit. Anom. 2013, 53, 9–17.
- Hu, W.Y.; Shi, G.B.; Hu, D.P.; Nelles, J.L.; Prins, G.S. Actions of Estrogens and Endocrine Disrupting Chemicals on Human Prostate Stem/Progenitor Cells and Prostate Cancer Risk. Mol. Cell. Endocrinol. 2012, 354, 63–73.
- Oral, D.; Erkekoglu, P.; Kocer-Gumusel, B.; Chao, M.W. Epithelial-Mesenchymal Transition: A Special Focus on Phthalates and Bisphenol A. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 43–58.
- Zhu, M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; Geng, S.; et al. Phthalates Promote Prostate Cancer Cell Proliferation through Activation of ERK5 and P38. Environ. Toxicol. Pharm. 2018, 63, 29–33.
- Park, M.-A.; Hwang, K.-A.; Lee, H.-R.; Yi, B.-R.; Jeung, E.-B.; Choi, K.-C. Cell Growth of BG-1 Ovarian Cancer Cells Is Promoted by Di-n-Butyl Phthalate and Hexabromocyclododecane via Upregulation of the Cyclin D and Cyclin-Dependent Kinase-4 Genes. Mol. Med. Rep. 2012, 5, 761–766.
- Grun, F.; Blumberg, B. Perturbed Nuclear Receptor Signaling by Environmental Obesogens as Emerging Factors in the Obesity Crisis. Rev. Endocr. Metab. Disord. 2007, 8, 161–171.
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H.; et al. Adverse Effects of Long-Term Exposure to Bisphenol A during Adulthood Leading to Hyperglycaemia and Hypercholesterolemia in Mice. Toxicology 2014, 325, 133–143.
- Li, J.; Lai, H.; Chen, S.; Zhu, H.; Lai, S. Gender Differences in the Associations between Urinary Bisphenol A and Body Composition among American Children: The National Health and Nutrition Examination Survey, 2003–2006. J. Epidemiol. 2017, 27, 228–234.
- Luzio, A.; Monteiro, S.M.; Rocha, E.; Fontainhas-Fernandes, A.A.; Coimbra, A.M. Development and Recovery of Histopathological Alterations in the Gonads of Zebrafish (Danio rerio) after Single and Combined Exposure to Endocrine Disruptors (17alpha-Ethinylestradiol and Fadrozole). Aquat. Toxicol. 2016, 175, 90–105.
- Young, B.J.; Lopez, G.C.; Cristos, D.S.; Crespo, D.C.; Somoza, G.M.; Carriquiriborde, P. Intersex and Liver Alterations Induced by Long-Term Sublethal Exposure to 17alpha-Ethinylestradiol in Adult Male Cnesterodon decemmaculatus (Pisces: Poeciliidae). Environ. Toxicol. Chem. 2016, 36, 1738–1745.
- Bernabo, I.; Biasone, P.; Macirella, R.; Tripepi, S.; Brunelli, E. Liver Histology and Ultrastructure of the Italian Newt (Lissotriton Italicus): Normal Structure and Modifications after Acute Exposure to Nonylphenol Ethoxylates. Exp. Toxicol. Pathol. 2014, 66, 455–468.
- Zhang, W.; Shen, X.Y.; Zhang, W.W.; Chen, H.; Xu, W.P.; Wei, W. The Effects of Di 2-Ethyl Hexyl Phthalate (DEHP) on Cellular Lipid Accumulation in HepG2 Cells and Its Potential Mechanisms in the Molecular Level. Toxicol. Mech. Methods 2017, 27, 245–252.
- Huff, M.; da Silveira, W.A.; Carnevali, O.; Renaud, L.; Hardiman, G. Systems Analysis of the Liver Transcriptome in Adult Male Zebrafish Exposed to the Plasticizer (2-Ethylhexyl) Phthalate (DEHP). Sci. Rep. 2018, 8, 2118.
- Radke, E.G.; Galizia, A.; Thayer, K.A.; Cooper, G.S. Phthalate Exposure and Metabolic Effects: A Systematic Review of the Human Epidemiological Evidence. Environ. Int. 2019, 132, 104768.
- Sun, Q.; Cornelis, M.C.; Townsend, M.K.; Tobias, D.K.; Eliassen, A.H.; Franke, A.A.; Hauser, R.; Hu, F.B. Association of Urinary Concentrations of Bisphenol A and Phthalate Metabolites with Risk of Type 2 Diabetes: A Prospective Investigation in the Nurses’ Health Study (NHS) and NHSII Cohorts. Environ. Health Perspect. 2014, 122, 616–623.
- Badamasi, I.; Odong, R.; Masembe, C. Threats Posed by Xenoestrogenic Chemicals to the Aquatic Ecosystem, Fish Reproduction and Humans: A Review. Afr. J. Aquat. Sci. 2020, 45, 243–258.
- Bhasker, C.R.; Hardiman, G. Advances in Pharmacogenomics Technologies. Pharmacogenomics 2010, 11, 481–485.
- Miracle, A.L.; Ankley, G.T. Ecotoxicogenomics: Linkages between Exposure and Effects in Assessing Risks of Aquatic Contaminants to Fish. Reprod. Toxicol. 2005, 19, 321–326.
- Martín-Díaz, M.L.; DelValls, T.A.; Riba, I.; Blasco, J. Integrative Sediment Quality Assessment Using a Biomarker Approach: Review of 3 Years of Field Research. Cell. Biol. Toxicol. 2008, 24, 513–526.
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342.
- Grün, F.; Blumberg, B. Endocrine Disrupters as Obesogens. Mol. Cell. Endocrinol. 2009, 304, 19–29.
- Baker, M.E.; Sprague, L.J.; Ribecco, C.; Ruggeri, B.; Lekmine, N.; Ludka, C.; Wick, I.; Soverchia, L.; Ubaldi, M.; Šášik, R.; et al. Application of a Targeted Endocrine Q-PCR Panel to Monitor the Effects of Pollution in Southern California Flatfish. Endocr. Disruptors 2014, 2, e969598.
- Baker, M.E.; Vidal-Dorsch, D.E.; Ribecco, C.; Sprague, L.J.; Angert, M.; Lekmine, N.; Ludka, C.; Martella, A.; Ricciardelli, E.; Bay, S.M.; et al. Molecular Analysis of Endocrine Disruption in Hornyhead Turbot at Wastewater Outfalls in Southern California Using a Second Generation Multi-Species Microarray. PLoS ONE 2013, 8, e75553.
- Hardiman, G. Microarray Technology—Advances, Applications, Future Prospects. Pharmacogenomics 2007, 8, 1639–1642.
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63.
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver From Short-Term Rat Toxicity Studies. Front. Genet. 2019, 9, 636.
- Casneuf, T.; Van de Peer, Y.; Huber, W. In Situ Analysis of Cross-Hybridisation on Microarrays and the Inference of Expression Correlation. BMC Bioinform. 2007, 8, 461.
- Chen, C.; Grennan, K.; Badner, J.; Zhang, D.; Gershon, E.; Jin, L.; Liu, C. Removing Batch Effects in Analysis of Expression Microarray Data: An Evaluation of Six Batch Adjustment Methods. PLoS ONE 2011, 6, e17238.
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969.
- Microarray Innovations: Technology and Experimentation. Available online: https://www.routledge.com/Microarray-Innovations-Technology-and-Experimentation/Hardiman/p/book/9780367385811 (accessed on 20 July 2021).
- Rouse, R.J.D.; Field, K.; Lapira, J.; Lee, A.; Wick, I.; Eckhardt, C.; Bhasker, C.R.; Soverchia, L.; Hardiman, G. Development and Application of a Microarray Meter Tool to Optimize Microarray Experiments. BMC Res. Notes 2008, 1, 45.
- Wick, I.; Hardiman, G. Biochip Platforms as Functional Genomics Tools for Drug Discovery. Curr. Opin. Drug Discov. Dev. 2005, 8, 347–354.
- Zhao, S.; Fung-Leung, W.P.; Bittner, A.; Ngo, K.; Liu, X. Comparison of RNA-Seq and Microarray in Transcriptome Profiling of Activated T Cells. PLoS ONE 2014, 9, e78644.
- Cloonan, N.; Forrest, A.R.; Kolle, G.; Gardiner, B.B.; Faulkner, G.J.; Brown, M.K.; Taylor, D.F.; Steptoe, A.L.; Wani, S.; Bethel, G.; et al. Stem Cell Transcriptome Profiling via Massive-Scale MRNA Sequencing. Nat. Methods 2008, 5, 613–619.
- Nagalakshmi, U.; Wang, Z.; Waern, K.; Shou, C.; Raha, D.; Gerstein, M.; Snyder, M. The Transcriptional Landscape of the Yeast Genome Defined by RNA Sequencing. Science 2008, 320, 1344–1349.
- da Silveira, W.A.; Hazard, E.S.; Chung, D.; Hardiman, G. Molecular Profiling of RNA Tumors Using High-Throughput RNA Sequencing: From Raw Data to Systems Level Analyses. Methods Mol. Biol. 2019, 1908, 185–204.
- Davis-Turak, J.; Courtney, S.M.; Hazard, E.S.; Glen, W.B.; da Silveira, W.A.; Wesselman, T.; Harbin, L.P.; Wolf, B.J.; Chung, D.; Hardiman, G. Genomics Pipelines and Data Integration: Challenges and Opportunities in the Research Setting. Expert Rev. Mol. Diagn. 2017, 17, 225–237.
- Rigden, D.J.; Fernández, X.M. The 2021 Nucleic Acids Research Database Issue and the Online Molecular Biology Database Collection. Nucleic Acids Res. 2021, 49, D1–D9.
- Lawler, M.; Siu, L.L.; Rehm, H.L.; Chanock, S.J.; Alterovitz, G.; Burn, J.; Calvo, F.; Lacombe, D.; Teh, B.T.; North, K.N.; et al. All the World’s a Stage: Facilitating Discovery Science and Improved Cancer Care through the Global Alliance for Genomics and Health. Cancer Discov. 2015, 5, 1133–1136.
- Leet, J.K.; Richter, C.A.; Cornman, R.S.; Berninger, J.P.; Bhandari, R.K.; Nicks, D.K.; Zajicek, J.L.; Blazer, V.S.; Tillitt, D.E. Effects of Early Life Stage Exposure of Largemouth Bass to Atrazine or a Model Estrogen (17α-Ethinylestradiol). PeerJ 2020, 8, e9614.
- Wang, J.; Zhou, J.; Yang, Q.; Wang, W.; Liu, Q.; Liu, W.; Liu, S. Effects of 17 α-Methyltestosterone on the Transcriptome, Gonadal Histology and Sex Steroid Hormones in Pseudorasbora Parva. Theriogenology 2020, 155, 88–97.
- Renaud, L.; Agarwal, N.; Richards, D.J.; Falcinelli, S.; Hazard, E.S.; Carnevali, O.; Hyde, J.; Hardiman, G. Transcriptomic Analysis of Short-Term 17α-Ethynylestradiol Exposure in Two Californian Sentinel Fish Species Sardine (Sardinops sagax) and Mackerel (Scomber japonicus). Environ. Pollut. 2019, 244, 926–937.
- Bertucci, A.; Pierron, F.; Gourves, P.-Y.; Klopp, C.; Lagarde, G.; Pereto, C.; Dufour, V.; Gonzalez, P.; Coynel, A.; Budzinski, H.; et al. Whole-Transcriptome Response to Wastewater Treatment Plant and Stormwater Effluents in the Asian Clam, Corbicula fluminea. Ecotoxicol. Environ. Saf. 2018, 165, 96–106.
- Legrand, E.; Forget-Leray, J.; Duflot, A.; Olivier, S.; Thomé, J.-P.; Danger, J.-M.; Boulangé-Lecomte, C. Transcriptome Analysis of the Copepod Eurytemora Affinis upon Exposure to Endocrine Disruptor Pesticides: Focus on Reproduction and Development. Aquat. Toxicol. 2016, 176, 64–75.
- Guo, J.; Mo, J.; Qi, Q.; Peng, J.; Qi, G.; Kanerva, M.; Iwata, H.; Li, Q. Prediction of Adverse Effects of Effluents Containing Phenolic Compounds in the Ba River on the Ovary of Fish (Hemiculter leucisculus) Using Transcriptomic and Metabolomic Analyses. Sci. Total Environ. 2021, 801, 149554.
- Garcia-Reyero, N. Are Adverse Outcome Pathways Here to Stay? Environ. Sci. Technol. 2015, 49, 3–9.
- Perkins, E.J.; Antczak, P.; Burgoon, L.; Falciani, F.; Garcia-Reyero, N.; Gutsell, S.; Hodges, G.; Kienzler, A.; Knapen, D.; McBride, M.; et al. Adverse Outcome Pathways for Regulatory Applications: Examination of Four Case Studies with Different Degrees of Completeness and Scientific Confidence. Toxicol. Sci. 2015, 148, 14–25.
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; LaLone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse Outcome Pathway (AOP) Development I: Strategies and Principles. Toxicol. Sci. 2014, 142, 312–320.
- What EDCs Are|Endocrine Society. Available online: https://www.endocrine.org/topics/edc/what-edcs-are (accessed on 20 July 2021).
