Periodontitis is a multifactorial chronic inflammatory disease that affects tooth-supporting soft/hard tissues of the dentition. The dental plaque biofilm is considered as a primary etiological factor in susceptible patients; however, other factors contribute to progression, such as diabetes and smoking. Current management utilizes mechanical biofilm removal as the gold standard of treatment. Antibacterial agents might be indicated in certain conditions as an adjunct to this mechanical approach. Studies suggest efficacy in the use of adjunctive antimicrobials in patients with grade C periodontitis of young age or where the associated risk factors are inconsistent with the amount of bone loss present. Meanwhile, alternative approaches such as photodynamic therapy and probiotics showed limited supportive evidence, and more studies are warranted to validate their efficiency.
- antibacterial
- biofilms
- periodontal debridement
- bacterial resistance
- photodynamic therapy
- periodontal disease
- probiotics
- periodontitis
1. Introduction
Periodontitis is an inflammatory disease initiated by dysbiosis of the subgingival microbiome, with aberrant immune response, causing collateral damage to the tooth-supporting tissues and ultimately leading to tooth loss [1][2]. Dental plaque biofilm is considered as the primary etiologic factor for the majority of dental/periodontal diseases [3]. The gold standard of treatment for periodontitis is mechanical debridement of subgingival biofilm. Indeed, suppression of pathogenic microorganisms has for a long time been a keystone in regeneration and repair of periodontal tissues, which can be challenging using mechanical debridement alone, which must be complemented by patient-based plaque control programs [4][5][6]. For many decades, attempts have been made to improve the efficacy of mechanical treatment by introducing different adjuncts such as the use of antimicrobials/antibiotics at different dosages and routes of administration. However, the structural complexity of dental biofilm provides a shelter for many pathogenic microorganisms, making delivery to individual bacteria challenging [7]. In addition, due to the non-specificity of these drugs, they may target useful commensal species which counteract pathogenic biofilm development [8].
2. Structure of Biofilm
3. Management of Dental Biofilm
3.1. Periodontal Debridement: The Gold Standard for Periodontal Therapy
3.2. Adjunctive Systemic and Local Antimicrobials/Antibiotics in Periodontics
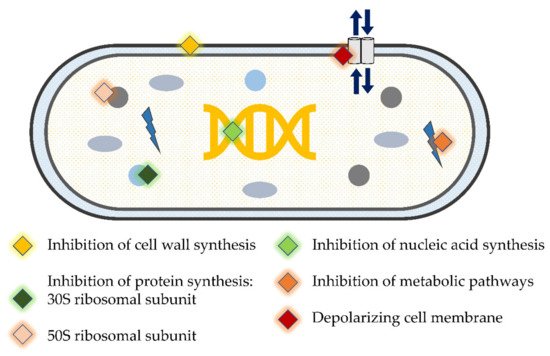
4. Using Antimicrobials/Antibiotics as Adjunct to Periodontal Therapy
A number of studies using various antimicrobials against periodontal pathogens have been carried out. It is apparent that the antimicrobials can kill periodontal pathogens in in vitro biofilm models. However, some studies indicated that amoxicillin (AMX) + metronidazole (MET) were not efficient in reducing the bacterial count [30][31]. Nevertheless, most of the studies consistently reported that the combination of these two antibiotics was superior to using either of them alone, particularly against red complex bacteria [32][33][34][35]. Similar results with regard to these bacteria were obtained with other antibiotics including azithromycin (AZM) [32][35], minocycline [36], and active organic ingredients of mouthrinses [37][38][39]. However, it is important to acknowledge that owing to greater tolerance to antimicrobials, the minimum inhibitory concentration calculated in in vitro studies would purportedly be lower and would bear little relevance to in vivo situations [18][40]. Furthermore, the majority of these studies used laboratory strains in their biofilm models, which apparently differ from clinical strains in their behavior and resistance to antimicrobials [41].
5. Novel Antibacterial Agents and Strategies to Overcome Bacterial Resistance in Dental Biofilm: Pros and Cons
| Author, Year | Study Design, Follow-Up | Study Population | Clinical/Microbiological Parameters | aPDT Treatment Modalities |
|---|---|---|---|---|
| Improvement in microbiological and clinical parameters § | ||||
| Moreira et al., 2015 [61] | Split-mouth RCT, 3-months | Patients with generalized AgP (n = 20) |
|
SD + Diode laser (670 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| Gandhi et al., 2019 [59] | Split-mouth, RCT, 9-months | Periodontitis patients (n = 26) |
|
SD + Diode laser (810 nm)/ICG photosensitizer |
| Annaji et al., 2016 [60] | Split-mouth RCT, 3-months | Patients with AgP (n = 15) |
|
SD+ Diode Laser (810 nm) |
| Wadhwa et al., 2021 [58] | Split-mouth RCT, 6-months | Chronic periodontitis patients (n = 30) | Total viable anaerobic count | SD + Diode laser (810 nm)/ICG photosensitizer |
| Improvement in microbiological parameters only § | ||||
| Muzaheed et al., 2020 [63] | Parallel arm RCT, 3-months | Periodontitis patients (n = 45) |
|
SD + Diode laser (660 nm)/methylene-blue (0.005%) photosensitizer |
| Chondros et al., 2009 [64] | Parallel arm RCT, 6-months | Periodontitis patients (n = 24) |
|
SD + Diode Laser (670 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| No improvement in microbiological and clinical parameters § | ||||
| Chitsazi et al., 2014 [70] | Split-mouth RCT, 3-months | Patients with AgP (n = 24) |
|
SD + Diode Laser (670–690 nm) |
| Rühling et al., 2010 [71] | Parallel arm RCT, 3-months | Periodontitis patients (n = 54) |
|
SD + Diode Laser (635 nm)/5% tolonium chloride photosensitizer |
| Queiroz et al., 2015 [68] Queiroz et al., 2014 [69] |
Parallel arm RCT, 3-months | Periodontitis smoker patients (n = 20) |
|
SD + Diode Laser (660 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| Tabenski et al., 2017 [66] | Parallel arm RCT, 12-months | Periodontitis patients (n = 45) |
|
SD + Diode Laser (670 nm)/phenothiazine chloride photosensitizer |
| Hill et al., 2019 [65] | Split-mouth RCT, 6-months | Periodontitis patients (n = 20) |
|
SD + Diode laser (808 nm)/ICG photosensitizer |
| Pulikkotil et al., 2016 [67] | Split-mouth RCT, 3-months | Periodontitis patients (n = 20) |
|
SD + LED lamp (red spectrum, 628 Hz)/methylene blue photosensitizer |
| Author, Year | Study Design, Follow-Up | Study Population | Strain of Probiotic | Mode/Frequency of Administration | Clinical/Microbiological Parameters |
|---|---|---|---|---|---|
| Improvement in microbiological and clinical parameters § | |||||
| Invernici et al., 2018 [72] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 41) | Bl (HN019) 1 × 109 CFU | Lozenges (10 mg) 2×/day for 30-days |
|
| Invernici et al., 2020 [73] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Bl (HN019) 1 × 109 CFU | Lozenges 2×/day in the morning and before bedtime for 30-days |
|
| Improvement in clinical parameters only § | |||||
| Laleman et al., 2020 [74] | Parallel arm RCT, 6-months | Chronic periodontitis patients (n = 39) | Lr (DSM 17,938 and ATCC PTA 5289) 2 × 108 CFU each | Five probiotic drops applied to residual pocket immediately after SD. Then each patient instructed to use lozenges 2×/day after brushing for 3-months |
|
| Tekce et al., 2015 [75] | Parallel arm RCT, 12-months | Chronic periodontitis patients (n = 30) | Lr (DSM 17,938 and ATCC PTA 5289) 2 × 108 CFU each | Lozenges 2×/day after brushing for 3-weeks |
|
| Improvement in microbiological parameters only § | |||||
| Dhaliwal et al., 2017 [76] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Sf (T-110 JPC), 30 × 107 CFU, Cb (TO-A HIS), 2 × 106 CFU, Bm (TO-A JPC), 1 × 106 CFU and Ls (HIS), 5 × 107 CFU | Bifilac lozenges 2×/day or 21-days |
|
| Teughels et al., 2013 [77] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Lr (DSM17938 and ATCC PTA5289) 9 × 108 CFU each | Lozenges 2×/day for 3-months |
|
| No improvement in microbiological and clinical parameters § | |||||
| Pudgar et al., 2021 [78] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 40) | Lb (CECT7480) and Lp (CECT7481), 6.0 × 109 CFU/mL each | One lozenge/day |
|
| Morales et al., 2018 [79] | Parallel arm RCT, 9-months | Chronic periodontitis patients (n = 47) | Lrh (SP1) 2 × 107 CFU | One sachet in water (150 mL) and ingest it once a day after brushing for 3-months |
|
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11010009
References
- Kirst, M.E.; Li, E.C.; Alfant, B.; Chi, Y.Y.; Walker, C.; Magnusson, I.; Wang, G.P. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl. Environ. Microbiol. 2015, 81, 783–793.
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontology 2000 2001, 25, 8–20.
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94.
- Buchmann, R.; Nunn, M.E.; Van Dyke, T.E.; Lange, D.E. Aggressive periodontitis: 5-year follow-up of treatment. J. Periodontol. 2002, 73, 675–683.
- Cugini, M.A.; Haffajee, A.D.; Smith, C.; Kent, R.L., Jr.; Socransky, S.S. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J. Clin. Periodontol. 2000, 27, 30–36.
- Carvalho, L.H.; D’Avila, G.B.; Leão, A.; Gonçalves, C.; Haffajee, A.D.; Socransky, S.S.; Feres, M. Scaling and root planing, systemic metronidazole and professional plaque removal in the treatment of chronic periodontitis in a Brazilian population II--microbiological results. J. Clin. Periodontol. 2005, 32, 406–411.
- Eick, S.; Seltmann, T.; Pfister, W. Efficacy of antibiotics to strains of periodontopathogenic bacteria within a single species biofilm—An in vitro study. J. Clin. Periodontol. 2004, 31, 376–383.
- Shlezinger, M.; Khalifa, L.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Resch, G.; Que, Y.A.; Beyth, S.; Dorfman, E.; Hazan, R.; Beyth, N. Phage Therapy: A New Horizon in the Antibacterial Treatment of Oral Pathogens. Curr. Top. Med. Chem. 2017, 17, 1199–1211.
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017.
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444.
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71–75.
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199.
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60.
- Mombelli, A. Maintenance therapy for teeth and implants. Periodontology 2000 2019, 79, 190–199.
- Suvan, J.E. Effectiveness of mechanical nonsurgical pocket therapy. Periodontology 2000 2005, 37, 48–71.
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56.
- Haffajee, A.D.; Teles, R.P.; Socransky, S.S. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontology 2000 2006, 42, 219–258.
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55.
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W.I. Investigation of a Novel Predictive Biomarker Profile for the Outcome of Periodontal Treatment. J. Periodontol. 2017, 88, 1135–1144.
- Saglie, F.R.; Marfany, A.; Camargo, P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J. Periodontol. 1988, 59, 259–265.
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350.
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontology 2000 2015, 67, 131–186.
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontology 2000 2017, 75, 152–188.
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501.
- Cionca, N.; Giannopoulou, C.; Ugolotti, G.; Mombelli, A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J. Periodontol. 2009, 80, 364–371.
- Cionca, N.; Giannopoulou, C.; Ugolotti, G.; Mombelli, A. Microbiologic testing and outcomes of full-mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. J. Periodontol. 2010, 81, 15–23.
- Feres, M.; Soares, G.M.; Mendes, J.A.; Silva, M.P.; Faveri, M.; Teles, R.; Socransky, S.S.; Figueiredo, L.C. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: A 1-year double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2012, 39, 1149–1158.
- Feres-Filho, E.J.; Silva, C.M.; Giovannetti-Menezes, N.; Torres, M.C.; Leão, A.T.; Sansone, C. Treatment of chronic periodontitis with systemic antibiotics only. J. Clin. Periodontol. 2006, 33, 936–937, author reply 931–940.
- Mombelli, A.; Almaghlouth, A.; Cionca, N.; Courvoisier, D.S.; Giannopoulou, C. Differential benefits of amoxicillin-metronidazole in different phases of periodontal therapy in a randomized controlled crossover clinical trial. J. Periodontol. 2015, 86, 367–375.
- Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Weiger, R.; Walter, C.; Zitzmann, N.U.; Waltimo, T. Microcalorimetric determination of the effects of amoxicillin, metronidazole, and their combination on in vitro biofilm. J. Periodontol. 2014, 85, 349–357.
- Belibasakis, G.N.; Thurnheer, T. Validation of antibiotic efficacy on in vitro subgingival biofilms. J. Periodontol. 2014, 85, 343–348.
- Soares, G.M.; Teles, F.; Starr, J.R.; Feres, M.; Patel, M.; Martin, L.; Teles, R. Effects of azithromycin, metronidazole, amoxicillin, and metronidazole plus amoxicillin on an in vitro polymicrobial subgingival biofilm model. Antimicrob. Agents Chemother. 2015, 59, 2791–2798.
- De Figueiredo, K.A.; da Silva, H.D.P.; Miranda, S.L.F.; Gonçalves, F.; de Sousa, A.P.; de Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Is as Effective as Amoxicillin in Controlling Red-Complex of Multispecies Subgingival Mature Biofilm In Vitro. Antibiotics 2020, 9, 432.
- Dabija-Wolter, G.; Al-Zubaydi, S.S.; Mohammed, M.M.A.; Bakken, V.; Bolstad, A.I. The effect of metronidazole plus amoxicillin or metronidazole plus penicillin V on periodontal pathogens in an in vitro biofilm model. Clin. Exp. Dent. Res. 2018, 4, 6–12.
- Ong, H.S.; Oettinger-Barak, O.; Dashper, S.G.; Darby, I.B.; Tan, K.H.; Reynolds, E.C. Effect of azithromycin on a red complex polymicrobial biofilm. J. Oral Microbiol. 2017, 9, 1339579.
- Schmid, J.L.; Kirchberg, M.; Sarembe, S.; Kiesow, A.; Sculean, A.; Mäder, K.; Buchholz, M.; Eick, S. In Vitro Evaluation of Antimicrobial Activity of Minocycline Formulations for Topical Application in Periodontal Therapy. Pharmaceutics 2020, 12, 352.
- Sánchez, M.C.; Llama-Palacios, A.; Marín, M.J.; Figuero, E.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. Validation of ATP bioluminescence as a tool to assess antimicrobial effects of mouthrinses in an in vitro subgingival-biofilm model. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e86–e92.
- Miranda, S.L.F.; Damaceno, J.T.; Faveri, M.; Figueiredo, L.C.; Soares, G.M.S.; Feres, M.; Bueno-Silva, B. In Vitro Antimicrobial Effect of Cetylpyridinium Chloride on Complex Multispecies Subgingival Biofilm. Braz. Dent. J. 2020, 31, 103–108.
- Ribeiro-Vidal, H.; Sánchez, M.C.; Alonso-Español, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812.
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776.
- Fux, C.A.; Shirtliff, M.; Stoodley, P.; Costerton, J.W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005, 13, 58–63.
- Harks, I.; Koch, R.; Eickholz, P.; Hoffmann, T.; Kim, T.S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; Doering, S.; et al. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J. Clin. Periodontol. 2015, 42, 832–842.
- Griffiths, G.S.; Ayob, R.; Guerrero, A.; Nibali, L.; Suvan, J.; Moles, D.R.; Tonetti, M.S. Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or re-treatment: A randomized controlled clinical trial. J. Clin. Periodontol. 2011, 38, 43–49.
- Cosgarea, R.; Eick, S.; Jepsen, S.; Arweiler, N.B.; Juncar, R.; Tristiu, R.; Salvi, G.E.; Heumann, C.; Sculean, A. Microbiological and host-derived biomarker evaluation following non-surgical periodontal therapy with short-term administration of systemic antimicrobials: Secondary outcomes of an RCT. Sci. Rep. 2020, 10, 16322.
- Mombelli, A.; Cionca, N.; Almaghlouth, A.; Décaillet, F.; Courvoisier, D.S.; Giannopoulou, C. Are there specific benefits of amoxicillin plus metronidazole in Aggregatibacter actinomycetemcomitans-associated periodontitis? Double-masked, randomized clinical trial of efficacy and safety. J. Periodontol. 2013, 84, 715–724.
- Mombelli, A.; Brochut, P.; Plagnat, D.; Casagni, F.; Giannopoulou, C. Enamel matrix proteins and systemic antibiotics as adjuncts to non-surgical periodontal treatment: Clinical effects. J. Clin. Periodontol. 2005, 32, 225–230.
- Pietruska, M.; Dolińska, E.; Milewski, R.; Sculean, A. Effect of systemic antibiotics on the outcomes of regenerative periodontal surgery in intrabony defects: A randomized, controlled, clinical study. Clin. Oral Investig. 2021, 25, 2959–2968.
- Loos, B.G.; Louwerse, P.H.; Van Winkelhoff, A.J.; Burger, W.; Gilijamse, M.; Hart, A.A.; van der Velden, U. Use of barrier membranes and systemic antibiotics in the treatment of intraosseous defects. J. Clin. Periodontol. 2002, 29, 910–921.
- Morales, A.; Contador, R.; Bravo, J.; Carvajal, P.; Silva, N.; Strauss, F.J.; Gamonal, J. Clinical effects of probiotic or azithromycin as an adjunct to scaling and root planning in the treatment of stage III periodontitis: A pilot randomized controlled clinical trial. BMC Oral Health 2021, 21, 12.
- Čuk, K.; Povšič, K.; Milavec, S.; Seme, K.; Gašperšič, R. Influence of adjunctive azithromycin on microbiological and clinical outcomes in periodontitis patients: 6-month results of randomized controlled clinical trial. BMC Oral Health 2020, 20, 241.
- Caffesse, R.G.; Sweeney, P.L.; Smith, B.A. Scaling and root planing with and without periodontal flap surgery. J. Clin. Periodontol. 1986, 13, 205–210.
- Oda, S.; Ishikawa, I. In vitro effectiveness of a newly-designed ultrasonic scaler tip for furcation areas. J. Periodontol. 1989, 60, 634–639.
- Stamatova, I.; Meurman, J.H. Probiotics and periodontal disease. Periodontology 2000 2009, 51, 141–151.
- Morozova, L.V.; Pozharitskaia, M.M.; Mel’nichuk, G.M. The therapeutic efficacy of probiotics for the correction of microfloral imbalance in periodontitis. Stomatologiia 1996, 68–69.
- Cobb, C.M. Lasers and the treatment of periodontitis: The essence and the noise. Periodontology 2000 2017, 75, 205–295.
- Dobson, J.; Wilson, M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch. Oral Biol. 1992, 37, 883–887.
- Giannelli, M.; Lasagni, M.; Bani, D. Photonic Therapy in Periodontal Diseases an Overview with Appraisal of the Literature and Reasoned Treatment Recommendations. Int. J. Mol. Sci. 2019, 20, 4741.
- Wadhwa, A.; Mallapragada, S.; Sharma, P. Novel indocyanine green mediated antimicrobial photodynamic therapy in the management of chronic periodontitis—A randomized controlled clinico-microbiological pilot study. J. Oral Biol. Craniofacial Res. 2021, 11, 57–62.
- Gandhi, K.K.; Pavaskar, R.; Cappetta, E.G.; Drew, H.J. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int. J. Periodontics Restor. Dent. 2019, 39, 837–843.
- Annaji, S.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Bharmappa, R.; Kamath, V. Efficacy of Photodynamic Therapy and Lasers as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis—A Clinical and Microbiologic Short Term Study. J. Clin. Diagn. Res. 2016, 10, ZC08–ZC12.
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386.
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102231.
- Muzaheed Acharya, S.; Hakami, A.R.; Allemailem, K.S.; Alqahtani, K.; Al Saffan, A.; Aldakheel, F.M.; Divakar, D.D. Effectiveness of single versus multiple sessions of photodynamic therapy as adjunct to scaling and root planing on periodontopathogenic bacteria in patients with periodontitis. Photodiagnosis. Photodyn. Ther. 2020, 32, 102035.
- Chondros, P.; Nikolidakis, D.; Christodoulides, N.; Rössler, R.; Gutknecht, N.; Sculean, A. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: A randomized controlled clinical trial. Lasers Med. Sci. 2009, 24, 681–688.
- Hill, G.; Dehn, C.; Hinze, A.V.; Frentzen, M.; Meister, J. Indocyanine green-based adjunctive antimicrobial photodynamic therapy for treating chronic periodontitis: A randomized clinical trial. Photodiagnosis. Photodyn. Ther. 2019, 26, 29–35.
- Tabenski, L.; Moder, D.; Cieplik, F.; Schenke, F.; Hiller, K.A.; Buchalla, W.; Schmalz, G.; Christgau, M. Antimicrobial photodynamic therapy vs. local minocycline in addition to non-surgical therapy of deep periodontal pockets: A controlled randomized clinical trial. Clin. Oral Investig. 2017, 21, 2253–2264.
- Pulikkotil, S.J.; Toh, C.G.; Mohandas, K.; Leong, K. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016, 61, 440–445.
- Queiroz, A.C.; Suaid, F.A.; de Andrade, P.F.; Oliveira, F.S.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.; Souza, S.L. Adjunctive effect of antimicrobial photodynamic therapy to nonsurgical periodontal treatment in smokers: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 617–625.
- Queiroz, A.C.; Suaid, F.A.; de Andrade, P.F.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.; Souza, S.L. Antimicrobial photodynamic therapy associated to nonsurgical periodontal treatment in smokers: Microbiological results. J. Photochem. Photobiol. B Biol. 2014, 141, 170–175.
- Chitsazi, M.T.; Shirmohammadi, A.; Pourabbas, R.; Abolfazli, N.; Farhoudi, I.; Daghigh Azar, B.; Farhadi, F. Clinical and Microbiological Effects of Photodynamic Therapy Associated with Non-surgical Treatment in Aggressive Periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 153–159.
- Rühling, A.; Fanghänel, J.; Houshmand, M.; Kuhr, A.; Meisel, P.; Schwahn, C.; Kocher, T. Photodynamic therapy of persistent pockets in maintenance patients-a clinical study. Clin. Oral Investig. 2010, 14, 637–644.
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210.
- Invernici, M.M.; Furlaneto, F.A.C.; Salvador, S.L.; Ouwehand, A.C.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.I.; Silva, P.H.F.; et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS ONE 2020, 15, e0238425.
- Laleman, I.; Pauwels, M.; Quirynen, M.; Teughels, W. A dual-strain Lactobacilli reuteri probiotic improves the treatment of residual pockets: A randomized controlled clinical trial. J. Clin. Periodontol. 2020, 47, 43–53.
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan Ipci, S.; Cakar, G.; Kadir, T.; Yılmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015, 42, 363–372.
- Dhaliwal, P.K.; Grover, V.; Malhotra, R.; Kapoor, A. Clinical and Microbiological Investigation of the Effects of Probiotics Combined with Scaling and Root Planing in the Management of Chronic Periodontitis: A Randomized, Controlled Study. J. Int. Acad. Periodontol. 2017, 19, 101–108.
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035.
- Pudgar, P.; Povšič, K.; Čuk, K.; Seme, K.; Petelin, M.; Gašperšič, R. Probiotic strains of Lactobacillus brevis and Lactobacillus plantarum as adjunct to non-surgical periodontal therapy: 3-month results of a randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 1411–1422.
- Morales, A.; Gandolfo, A.; Bravo, J.; Carvajal, P.; Silva, N.; Godoy, C.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.; Gamonal, J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: A randomized placebo- controlled trial with 9-month follow-up. J. Appl. Oral Sci. 2018, 26, e20170075.
