While water shortage across the world is threatening the well-being of the human community, emerging advanced technologies targeted to address this challenge are promising. In this regard, nanomaterials have played a crucial role. Nanomaterials, i.e., those materials which have at least one dimension in the 1–100 nm size range, have produced a new generation of technologies for water purification. This includes nanosized adsorbents, nanomembranes, photocatalysts, etc. Stemming from extraordinary structural characteristics and size scale of nanomaterials, the nanostructured membranes/adsorbents enable water purification with a high efficiency in terms of pollutants removal and water permeability, thereby reducing energy consumption and cost.
- nanomaterials
- Water treatment
- membrane
- adsorbent
- photocatalyst
1. Introduction
Water, a previously plentiful, free resource across the world, has become a rare, costly object over recent decades and currently, water shortage is going to be a challenge for sustainable development of the human community [1][2]. This crisis is dramatically expanding and is regarded a global systemic risk, mainly resulting from urban, agricultural, and industrial pollution. In these areas, water consumption has incremented up to 70% (agriculture), 22% (industry), and 8% (domestic) of the currently available fresh water and, accordingly, an enormous volume of wastewater containing a variety of pollutants has been produced [3]. No doubt, the release of wastewater from commercial and industrial sectors besides untreated domestic sewage and chemical pollutants into fresh water resources is horribly detrimental to both the human community and the ecosystem, including animals and plants. In this regard, the major water contaminants are heavy metal ions, organics (e.g., dyes), and oils that can disqualify any water stream for drinking.
To address the need for water remediation systems, during the past few decades, with the evolution of nanotechnology, a diverse range of new technologies based on nanomaterials has been developed. For instance, as adsorbent systems, nanomaterials offer an extremely large reactive surface area at a low mass, can be produced at a much less cost compared to activated carbon and they can remove pollutants efficiently [2]. In this regard, a plethora of nano-adsorbents in various forms and dimensionalities (D) like nanoparticles (0D), nanofibers and nanotubes (1D), nanosheets (2D), and nanoflowers (3D) has been investigated [2]. In terms of composition, the diversity is indeed extreme and many organic and inorganic nanomaterials have been synthesized that can help purify water streams. The separation mechanism can be based on chemical/physical affinity of the pollutant for the surface of the nanomaterial or through size exclusion of the pollutant by a porous nanomaterial system. In the latter case, nanomaterials act either as the main building block of the porous separator structure, as seen for nanofibrous microfiltration or ultrafiltration membranes, or as an additive to a polymeric thin film membrane to improve its hydrophilicity and thermomechanical properties. The previously mentioned separation processes such as adsorption or filtration only gather the pollutant molecules in solid form but never entirely “eliminate” or “decompose” them. This issue could be problematic because disposal of the obtained sludge and fouling of the filtration system is challenging [4]. For such reasons, the separation process should be complemented by degradation processes such as photocatalysis, sonocatalysis, and reductive degradation that allow the decomposition of organic pollutants into non-toxic metabolites. Other than environmental remediation, nanomaterials can also be efficiently applied for environmental control and construction of sensors that can detect even trace amounts of water pollutants.
2. Nanomaterials for Water Purification
As mentioned earlier, nanomaterials offer several advantages for water treatment and control. This amazing potential stems from their large exposed surface area and functionality that can maximize their interactivity with water pollutants.
2.1. Nanomaterials for Adsorption and Photodecomposition
In the water treatment field, the removal of dye pollutants due to their acute toxicities and carcinogenic nature is of paramount importance. Dyes have a history of thousands of years of application for textiles, paints, pigments, etc. Currently, almost 100,000 types of dyes are produced commercially. In terms of consumption volume, approximately 1.6 million tons of dyes are consumed annually. Thereof, 10–15% are wasted during use [5]. The dye pollutants released from industrial and agricultural wastes are refractory and potentially present carcinogenic effects. Therefore, they must be excluded from water streams through different kinds of traditional treatments, such as activated sludge, chemical coagulation, adsorption, and photocatalytic degradation [6]. Superior to the mentioned techniques, adsorption is relatively effective in the creation of a high quality effluent with no harmful byproducts in an energy/cost efficient manner [7][8][9]. This approach allows for exclusion of soluble and insoluble organic, inorganic, and biological water contaminants. The diverse merits of adsorption for dye removal are convincing enough to devise sustainable adsorbents that are manufactured on a large scale at low cost and enable fast and efficient dye removal. For this sake, within the course of the past few decades and with the evolution of nanotechnology, a variety of adsorbents of nanoscale size have been scrutinized. Nanomaterials provide an extensive reactive surface area at a low mass and versus activated carbon, i.e., the golden benchmark of adsorbents, they can be produced in a less expensive manner while removing dyestuffs and organic pollutants with a notably less amount [2]. Some examples of dye nano-adsorbents are as follow. Chitosan-coated magnetite (Fe3O4) nanoparticles showed a large adsorption capacity for crocein orange G (1883 mg/g) and acid green 25 (1471 mg/g). Interestingly, the adsorbent nanoparticles could be readily recovered by a magnetic field [10]. Based on such a concept, Fe3O4/activated carbon nanoparticles (6–16 nm) that can separate 138 and 166.6 mg/g methylene blue (MB) and brilliant green dyes, respectively, have been developed [11]. Dhananasekaran et al. [12] synthesized α-chitin nanoparticles (< 50 nm) from Penaeus monodon shell waste and tested their dye (methylene blue (MB), bromophenol blue (BPB), and Coomassie brilliant blue (CBB)) adsorption efficiency. The nanoparticle adsorbent showed an adsorption efficiency of 95.96–99%, depending on the adsorbent concentration and based on physical adsorption of the dyestuff to the nanoparticles. Other than nanoparticles, nanofibrous adsorbents have also found application in the removal of dye pollutants from water. Such adsorbents are typically made through electrospinning and are superior to nanoparticulate adsorbents due to their easy recovery. As an example for nanofibrous adsorbents, polyethersulfone (PES) electrospun nanofibers containing V2O5 nanoparticles have been employed for removal of MB dye pollutant from water [13]. The nanocomposite nanofibers show a low isoelectric point thus at elevated pHs they acquire an extensive highly hydroxylated surface area that facilitates adsorption of cationic MB molecules.
One drawback of adsorption is its inability to completely “eliminate” or “decompose” the dye pollutants. Instead, it solely collects the dye molecules by transferring them to other phases. This feature could be challenging due to the fact that discharge of the dye-related sludge is not straightforward and the adsorbent is rarely reusable [4]. Accordingly, there is a need to complementary degrading treatments such as photocatalysis, sonocatalysis, and reductive degradation that enable decomposition of dye to non-toxic metabolites. In this regard, a variety of advanced oxidation processes (AOPs), provoking release of hydroxyl radicals (OH•), have shown a promising potential for decolorization of textile effluents. With unpaired electrons, OH• is drastically reactive and oxidizes recalcitrant organic pollutants [14]. Due to the abundance of low cost, while operative photocatalysts, photocatalysis is indeed of the most researched AOP processes and is considered as a practical degradation process for organic dyes and pesticides. Various semiconductor metal oxide nanoparticles such as ZnO and TiO2 have shown notable efficiency in the photodecomposition of dye pollutants. For instance, Li et al. [15]developed an oil-in-water Pickering emulsion (PE) stabilized by the presence of TiO2 particles, wherein the dye containing wastewater and insoluble organic matter were regarded as the water and oil phases, respectively. The TiO2 particles could offer a large photoactivity effect and notably degrade the dye molecules (Figure 1a). In another relevant study, Kheirabadi et al. [16]synthesized a ternary nanostructure composed of Ag nanoparticle/ZnO nanorod/3-dimensional graphene (3DG) network via a coupled hydrothermal-photodeposition technique. While the 3DG can capture 300 mg/g MB dye by an adsorption process, Ag/ZnO component brings about the possibility of photodecomposition of the dye even under visible light irradiation. The dye removal mechanism of the synthesized adsorbent/photocatalytic system is illustrated in Figure 1b.
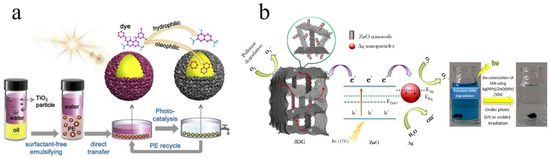
Figure 1. (a) Schematic illustration of an oil/water Pickering emulsion (PE) consisting superhydrophilic TiO2 particles enabling dye photodecomposition. Reproduced with permission from [15]. Copyright 2019, Elsevier. (b) The dye removal mechanism of an adsorbent/photocatalyst system comprising Ag nanoparticle/ZnO nanorod/3D-graphene hydrogel. Reproduced with permission from [16]. Copyright 2019, Elsevier.
2.2. Nanomaterials for Membrane-Based Water Treatment
A membrane is a selective barrier located between two homogenous phases that splits a feed water stream into a retentate and a permeate fraction. The pressure difference between the feed and permeate sides acts as the driving force for the membrane’s action and passes water through the membrane [17]. As a result, based on charge, size, and shape, solutes and particles are discriminated (Figure 2a). The new generation of membrane technologies employ nanomaterials for water treatment.
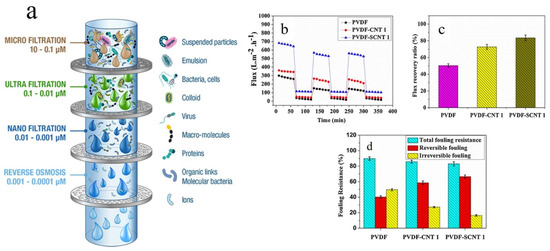
Figure 2. (a) Schematic shows diverse membrane filtration processes including reverse osmosis, nanofiltration, ultrafiltration, microfiltration, and particle filtration. Other than the reverse osmosis membranes whose structure is almost dense and non-porous, the rest are different in terms of the average pore size (the image was obtained from www.muchmorewater.com). Antifouling properties of CNT/PVDF UF membranes represented in cycle filtration (b), water recovery flux (c), and fouling resistance (d). Reproduced with permission from [18]. Copyright 2019, Elsevier.
Nanocomposite membranes comprising a thin polymeric film surface decorated or incorporated with nanofillers are a distinguished class of membranes able to dynamically purify water. Nanomaterials in different forms and dimensionalities can be used in construction of nanocomposite membranes. Nanoparticles, for instance, have been widely used as nanofillers for mechanical reinforcement or for hydrophilization of polymeric membranes. In this regard, Rodrigues et al. [19] incorporated clay nanoparticles into mixed matrix polysulfone ultrafiltration membranes to improve thermomechanical properties and water permeability of the membrane, while maintaining optimum rejection efficiency. Moreover, the membranes reinforced with clay nanoparticles showed a lower fouling tendency and higher flux recovery when tested with sodium alginate and natural water. One critical concern regarding ultrafiltration (UF) membranes is their biofouling and the presence of bacterial colonies on the surface and thereby clogging the pores and lowering the permeability of the membrane. In this regard, extracellular polymeric substances (EPS) are released upon bacterial cell lysis and are adsorbed on the UF membrane and thus reduce the longevity and permeability of the membrane [18][20]. The most promising solution to address the challenge of biofouling of the UF membranes is surface hydrophilization by incorporation of various antifouling agents [21]. In this regard, a diverse range of antifouling agents has been employed in membrane technology, including Ag, Au, Cu, graphene oxide (GO), Zn, and TiO2 nanoparticles [22][23], and also carbon nanotubes (CNTs) [18]. Despite the significance of industrial production of such nanocomposite membranes for water treatment, their toxicity that could originate from the release of the incorporated nanomaterials during the high pressure difference-driven filtration process should be carefully evaluated to minimize their adverse effects on human health and the environment [24]. The toxicity profile of the nanoparticles embedded in a polymeric matrix could be a function of their size, shape, charge and preparation conditions [18]. Among the nanofillers above mentioned, CNTs are resilient antibacterial agents whose toxic effect is derived from the ions and reactive oxygen species (ROSs) they release and thereby kill bacteria through oxidative stress stimuli [25]. Such a remarkable performance has led to wide application of CNTs in blended UF membranes, for the sake of improvement of filtration performance [26]. As reported in many studies, CNTs optimize water filtration and rejection of salts, nonpolar contaminants, micro- and macro-sized contaminants, and also waste chemical materials [27]. Ayyaru et al. [18] synthesized CNT- and sulphonated CNT (SCNT)-blended UF polyvinylidene fluoride (PVDF) membranes. For the latter group of the membranes, bovine serum albumin (BSA) rejection was 90%. As shown in Figure 2b, flux decline was less notable for the SCNT-PVDF membrane while permeating BSA solution through the membranes, thanks to its improved hydrophilicity. For the CNT- and SCNT-PVDF membranes, the fouling recovery ratio (FRR) was 72.74 and 83.52%, respectively, Figure 2c, implying their optimum antifouling effect arisen from –SO3H and –OH groups found in SCNT and CNT, respectively. According to Figure 2d, the irreversible fouling value of the nanocomposite membranes, particularly that of SCNT-PVDF, is lower than that of the neat PVDF membrane. This again emphasizes the role of hydrophilicity induced by the presence of the nanofillers on lowering the fouling tendency of the membranes. Although CNTs are potentially versatile additives to membranes and also promising adsorbents for divalent metal ions, dyes, natural organic matters, etc., their relatively high unit cost is a limiting factor for their widespread practical use [3]. Moreover, the existence of metal catalysts in raw CNTs might induce a toxic effect. In contrast, chemically functionalized CNTs have not been shown yet to be toxic [28]. Accordingly, practical applicability of CNTs as adsorbents or inclusions in membranes for water treatment is tightly associated with finding cost effective production methods for CNTs and minimizing their toxicity effect by development of safer alternatives such as carbon nanocrystals (CNCs) [3].
3. Conclution
Nanomaterials are favorable candidate materials for water remediation and control and an exciting prospect for their integration into point-of-use systems, and also in absolute removal of the current and emerging inorganic and organic pollutants from water is foreseen. They can be used in construction of nanoadsorbents that effectively capture polar and non-polar pollutants from water depending on their surface functionality. In this regard, the extensive surface area offered by nanomaterials is decisive and maximizes the adsorption efficiency. In case, the used nanomaterial is a photocatalyst, adsorption is extended to photodecomposition. Accordingly, the stuck organic pollutant is degraded into harmless byproducts and the adsorbent’s surface becomes ready for a next round of adsorption/photodecomposition process. As another opportunity originated from nanomaterials, membrane nanostructures can be mentioned. Nanomaterials can be exploited as building blocks of a porous separator, as seen in electrospun nanofibrous membranes or single/few layer graphene membranes. Additionally, they can be used as additives to conventional thin film polymeric membranes for ultrafiltration to reduce fouling tendency and to raise thermomechanical properties.
This entry is adapted from the peer-reviewed paper 10.3390/w12041150
References
- Homaeigohar, S.; Elbahri, M. Nanocomposite Electrospun Nanofiber Membranes for Environmental Remediation. Materials 2014, 7, 1017–1045.
- Homaeigohar, S. The Nanosized Dye Adsorbents for Water Treatment. Nanomaterials 2020, 10, 295.
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137, doi:10.1016/j.cej.2016.08.053.
- Bansal, P.; Chaudhary, G.R.; Mehta, S.K. Comparative study of catalytic activity of ZrO2 nanoparticles for sonocatalytic and photocatalytic degradation of cationic and anionic dyes. Chem. Eng. J. 2015, 280, 475–485, doi:10.1016/j.cej.2015.06.039.
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242.
- Shan, G.; Surampalli, R.Y.; Tyagi, R.D.; Zhang, T.C. Nanomaterials for environmental burden reduction, waste treatment, and nonpoint source pollution control: A review. Front. Environ. Sci. Eng. China 2009, 3, 249–264.
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085.
- Homaeigohar, S.; Elbahri, M. An Amphiphilic, Graphitic Buckypaper Capturing Enzyme Biomolecules from Water. Water 2019, 11, 2.
- Homaeigohar, S.; Strunskus, T.; Strobel, J.; Kienle, L.; Elbahri, M. A Flexible Oxygenated Carbographite Nanofilamentous Buckypaper as an Amphiphilic Membrane. Adv. Mater. Interfaces 2018, 5, 1800001, doi:doi:10.1002/admi.201800001.
- Chang, Y.C.; Chen, D.H. Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan‐conjugated magnetic nano‐adsorbent. Macromol. Biosci. 2005, 5, 254–261.
- Joshi, S.; Garg, V.K.; Kataria, N.; Kadirvelu, K. Applications of Fe3O4@AC nanoparticles for dye removal from simulated wastewater. Chemosphere 2019, 236, 124280, doi:10.1016/j.chemosphere.2019.07.011.
- Dhananasekaran, S.; Palanivel, R.; Pappu, S. Adsorption of Methylene Blue, Bromophenol Blue, and Coomassie Brilliant Blue by α-chitin nanoparticles. J. Adv. Res. 2016, 7, 113–124, doi:10.1016/j.jare.2015.03.003.
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848.
- Hisaindee, S.; Meetani, M.; Rauf, M. Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. Trac Trends Anal. Chem. 2013, 49, 31–44.
- Li, Q.; Zhao, T.; Li, M.; Li, W.; Yang, B.; Qin, D.; Lv, K.; Wang, X.; Wu, L.; Wu, X.; et al. One-step construction of Pickering emulsion via commercial TiO2 nanoparticles for photocatalytic dye degradation. Appl. Catal. B: Environ. 2019, 249, 1–8, doi:10.1016/j.apcatb.2019.02.057.
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid Interface Sci. 2019, 537, 66–78, doi:10.1016/j.jcis.2018.10.102.
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. Npg Asia Mater. 2017, 9, e427.
- Ayyaru, S.; Pandiyan, R.; Ahn, Y.-H. Fabrication and characterization of anti-fouling and non-toxic polyvinylidene fluoride -Sulphonated carbon nanotube ultrafiltration membranes for membrane bioreactors applications. Chem. Eng. Res. Des. 2019, 142, 176–188, doi:10.1016/j.cherd.2018.12.008.
- Rodrigues, R.; Mierzwa, J.C.; Vecitis, C.D. Mixed matrix polysulfone/clay nanoparticles ultrafiltration membranes for water treatment. J. Water Process Eng. 2019, 31, 100788, doi:10.1016/j.jwpe.2019.100788.
- Ayyaru, S.; Choi, J.; Ahn, Y.-H. Biofouling reduction in a MBR by the application of a lytic phage on a modified nanocomposite membrane. Environ. Sci. Water Res. Technol. 2018, 4, 1624–1638.
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723.
- Homaeigohar, S.S.; Mahdavi, H.; Elbahri, M. Extraordinarily water permeable sol gel formed nanocomposite nanofibrous membranes. J. Colloid Interface Sci. 2012, 366, 51–56.
- Homaeigohar, S.; Botcha, N.K.; Zarie, E.S.; Elbahri, M. Ups and Downs of Water Photodecolorization by Nanocomposite Polymer Nanofibers. Nanomaterials 2019, 9, 250.
- Yong, C.W. Study of interactions between polymer nanoparticles and cell membranes at atomistic levels. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140036.
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. Acs Nano 2010, 4, 5731–5736.
- Al-Khaldi, F.A.; Abusharkh, B.; Khaled, M.; Atieh, M.A.; Nasser, M.; Saleh, T.A.; Agarwal, S.; Tyagi, I.; Gupta, V.K. Adsorptive removal of cadmium (II) ions from liquid phase using acid modified carbon-based adsorbents. J. Mol. Liq. 2015, 204, 255–263.
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109.
- Chen, C.; Wang, X. Adsorption of Ni (II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind. Eng. Chem. Res. 2006, 45, 9144–9149.
