Algae taxa are notably diverse regarding pigment diversity and composition, red seaweeds (Rhodophyta) being a valuable source of phycobiliproteins (phycoerythrins, phycocyanin, and allophycocyanin), carotenes (carotenoids and xanthophylls), and chlorophyll a. These pigments have a considerable biotechnological potential, which has been translated into several registered patents and commercial applications.
- Rhodophyta
- phycobilins
- chlorophylls
- carotenoids
- pigment
1. Phycobiliproteins
1.1. Distribution, Properties and Structure
1.2. Biotechnological Potential and Applications
PBPs have noteworthy spectroscopic properties, such as high absorption coefficient, high excitation and emission spectra, high quantum yield, low interference, high quenching stability, and water solubility [9][18]. Therefore, they have been widely considered in several and well documented applications, namely, in biomedical research, clinical diagnostics, therapeutic science, and cosmeceutical and pharmaceutical industries [4][14][19][20].
1.3. Extraction and Purification Methods
PBPs are not easy and straightforward obtained. Traditionally, the methods to obtain PBP extracts present a challenge by themselves since, as mentioned, PBP are located within the phycobilissome inside the chloroplast, and thus, the algae must be pretreated with appropriate solvents, and the cells must be homogenized and disrupted using suitable methods, to release the contents within [2]; this must be achieved while avoiding any step or method that involves high temperatures, as these pigments are highly thermosensitive.
There are different methods to perform the extraction, whose choice is a critical step to attain a maximum PBP recover, as it has a significant impact on activity and purity of the obtained pigment [4]. Specific factors include biomass conditioning (fresh versus dry algae), biomass/solvent ratio, cellular disruption method, solvent, extraction time and number of steps taken [2], storage method prior to extraction [39], and even factors unconnected to the methodology itself, such as the harvest season [2], and species where the pigment was extracted from [40].
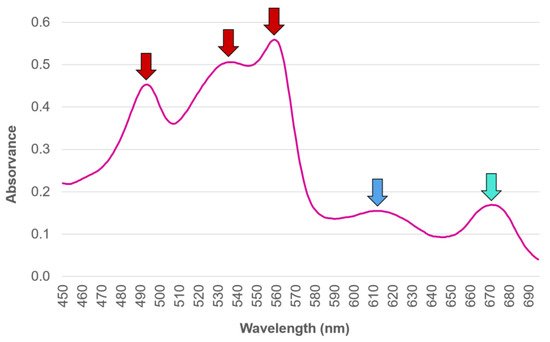
Traditionally, the abundance and diversity of the different PBP in an organism has been commonly estimated by means of light absorption assessment at distinct wavelengths, performed upon the PBP extracted from the biological matrix [41][42]. However, authors such as Saluri et al. [43], who lists several other works that reply on this approach to assess the PBP R-PE (a subtype of PE obtained from Rhodophyta [14]) content in red seaweed species, defend a more reliable method being needed, so that the most promising algae species regarding R-PE content can be targeted. According to the authors, the traditional method of absorbance reading is both quick and widely used; however, it can produce misleading results whenever impurities remain present in the samples, and it is unreliable overall, regardless of whether it is assessed upon crude extracts or following their purification. An example offered in order to circumvent this is found in Saluri et al. [43]’s work, which describes an alternative approach of employing the High Performance Liquid Chromatography technique of Size Exclusion Chromatography (HPLC-SEC) method with fluorescence and photo-diode array detectors, not only to separate PBP from interfering compounds, but also to reliably quantify their yields.
1.4. Production and Commercialization
As the production of PBP from natural sources requires a high investment in large-scale cyanobacteria or algae cultures, alternative approaches to obtain this pigment for biotechnological purposes has been investigated. Specifically, the production of recombinant PBP in Escherichia coli, while retaining all the qualities of a pigment obtained from native organisms, has been studied by a number of authors [20][44][45].
Nevertheless, a few examples can be found on a commercial level, where there are a few companies profiting from PBP commercialization in the form of natural dyes yet featuring prominently microalgae as the source for their products. For example, the commercially available blue colorant Linablue® Spirulina Extract is sold as a food product decoration component, and several companies promote their microalgae-based natural dyes to be introduced in cosmetic products, namely, phycocyanin from Arthrospira sp.
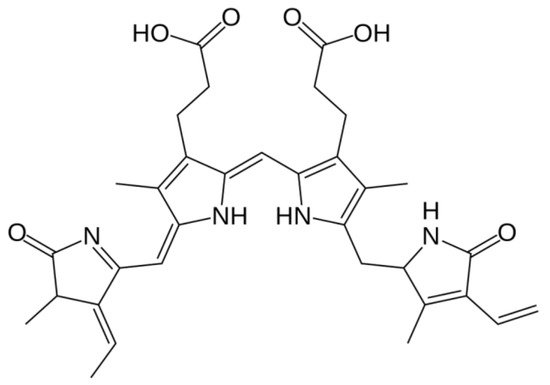
1.5. Phycoerythrin
1.6. Phycocyanin
1.7. Allophycocyanin
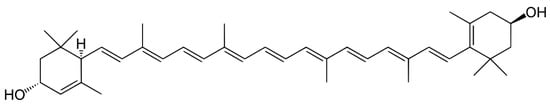
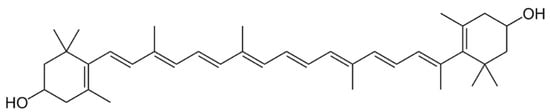
2. Carotenoids
2.1. Distribution, Properties and Structure
2.2. Biotechnological Potential and Applications
Carotenoids enhance the nutritional value of a myriad of natural sources, such as fruit and vegetables, eggs, fish, algae, fungi, and yeasts [67][68]. In the human diet, approximately 50 carotenoids can be found [66]. Additionally, carotenoids are highly acknowledged in the cosmetic industry, by improving skin health by increasing dermal defense against UV [63]. They act as antioxidant agents, working connected with other reducing agents such as polyphenols and vitamins (C and E) [69]. Carotenoids also aid in enhancing immune defenses, and have a role in reducing the incidence of chronic diseases and diseases associated with aging, such as inflammatory diseases, cardiovascular diseases, bone/skin/eye disorders, diabetes, and cancer [62][63][70], while also promoting mental and metabolic health in both pregnancy and early life [70]. A number of carotenoids combat vitamin A deficiencies, due to their ability to be converted into retinoids that exhibit vitamin A activity [68]. Additionally, they are sources of color, odor, and taste [71].
2.3. Carotenes
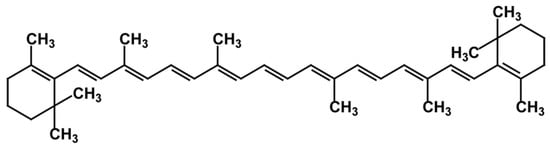
2.4. Xanthophylls
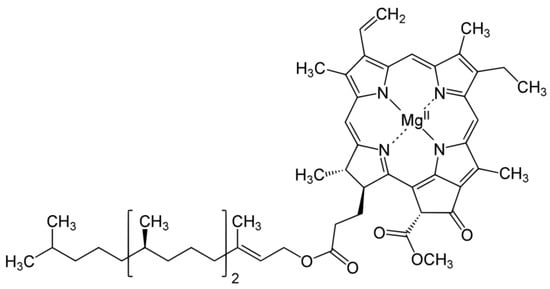
3. Chlorophyll
3.1. Distribution, Properties and Structure
3.2. Biotechnological Potential and Applications
3.3. Extraction and Purification Methods
This entry is adapted from the peer-reviewed paper 10.3390/phycology2010001
References
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600.
- Beattie, S.W.; Morançais, M.; Déléris, P.; Fleurence, J.; Dumay, J. Extraction of phycocyanin and phycoerythrin pigments. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 249–265.
- Sonani, R.R. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100.
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353.
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed Res. Int. 2013, 2013, 742859.
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Phycoerythrins: Valuable proteinic pigments in red seaweeds. Adv. Bot. Res. 2014, 71, 321–343.
- Cohen-Bazire, G.; Bryant, D.A. Phycobilisomes: Composition and Structure. In The Biology of Cyanobacteria; Carr, N.G., Whitton, B.A., Eds.; Blackwell Publishing: Oxford, UK, 1982; pp. 143–190.
- Román, R.B.; Alvárez-Pez, J.M.; Fernández, F.G.A.; Grima, E.M. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85.
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in Phycobiliproteins Research: Innovations and Commercialization. In Natural Bioactive Compounds: Technological Advancements; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: London, UK, 2021; pp. 57–81.
- Basheva, D.; Moten, D.; Stoyanov, P.; Belkinova, D.; Mladenov, R.; Teneva, I. Content of phycoerythrin, phycocyanin, alophycocyanin and phycoerythrocyanin in some cyanobacterial strains: Applications. Eng. Life Sci. 2018, 18, 861–866.
- Zhao, M.; Sun, L.; Fu, X.; Chen, M. Phycoerythrin-phycocyanin aggregates and phycoerythrin aggregates from phycobilisomes of the marine red alga Polysiphonia urceolata. Int. J. Biol. Macromol. 2019, 126, 685–696.
- Chen, H.; Jiang, P. Combinational biosynthesis and characterization of fusion proteins with tandem repeats of allophycocyanin holo-α subunits, and their application as bright fluorescent labels for immunofluorescence assay. J. Biosci. Bioeng. 2018, 126, 778–782.
- Adir, N.; Bar-Zvi, S.; Harris, D. The amazing phycobilisome. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148047.
- Fleurence, J. R-phycoerythrin from red macroalgae: Strategies for extraction and potential application in biotechnology. Appl. Biotechnol. Food Sci. Policy 2003, 1, 1–6.
- Talarico, L.; Maranzana, G. Light and adaptive responses in red macroalgae: An overview. J. Photochem. Photobiol. B Biol. 2000, 56, 1–11.
- Wehrmeyer, W. Phycobilisomes: Structure and Function. In Experimental Phycology: Cell Walls and Surfaces, Reproduction, Photosynthesis; Wiesser, W., Robinson, D.G., Starr, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 158–172.
- López-Figueroa, F. Diurnal Variation in Pigment Content in Porphyra laciniata and Chondrus crispus and its Relation to the Diurnal Changes of Underwater Light Quality and Quantity. Mar. Ecol. 1992, 13, 285–305.
- Niu, J.; Xu, M.; Wang, G.; Zhang, K.; Peng, G. Comprehensive extraction of agar and R-phycoerythrin from Gracilaria lemaneiformis (Bangiales, Rhodophyta). Indian J. Mar. Sci. 2013, 42, 21–28.
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443.
- Dagnino-Leone, J.; Figueroa, M.; Uribe, E.; Hinrichs, M.V.; Ortiz-López, D.; Martínez-Oyanedel, J.; Bunster, M. Biosynthesis and characterization of a recombinant eukaryotic allophycocyanin using prokaryotic accessory enzymes. Microbiologyopen 2020, 9, e989.
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266.
- Hemlata; Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89.
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during In Vitro digestion. Food Sci. Technol. Res. 2010, 16, 347–352.
- Nagaraj, S.; Arulmurugan, P.; Rajaram, M.G.; Karuppasamy, K.; Jayappriyan, K.R.; Sundararaj, R.; Vijayanand, N.; Rengasamy, R. Hepatoprotective and antioxidative effects of C-phycocyanin from Arthrospira maxima SAG 25780 in CCl4-induced hepatic damage rats. Biomed. Prev. Nutr. 2012, 2, 81–85.
- Pardhasaradhi, B.V.V.; Mubarak Ali, A.; Leela Kumari, A.; Reddanna, P.; Khar, A. Phycocyanin-mediated apoptosis in AK-5 tumor cells involves down-regulation of Bcl-2 and generation of ROS. Mol. Cancer Ther. 2003, 2, 1165–1170.
- Reddy, M.C.; Subhashini, J.; Mahipal, S.V.K.; Bhat, V.B.; Reddy, P.S.; Kiranmai, G.; Madyastha, K.M.; Reddanna, P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 385–392.
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of Phycocyanin. J. Funct. Foods 2014, 11, 375–392.
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14.
- Shih, S.R.; Tsai, K.N.; Li, Y.S.; Chueh, C.C.; Chan, E.C. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J. Med. Virol. 2003, 70, 119–125.
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521.
- Lee, P.T.; Yeh, H.Y.; Lung, W.Q.C.; Huang, J.; Chen, Y.J.; Chen, B.; Nan, F.H.; Lee, M.C. R-phycoerythrin from Colaconema formosanum (Rhodophyta), an anti-allergic and collagen promoting material for cosmeceuticals. Appl. Sci. 2021, 11, 9425.
- Hao, S.; Li, S.; Wang, J.; Zhao, L.; Zhang, C.; Huang, W.; Wang, C. Phycocyanin Reduces Proliferation of Melanoma Cells through Downregulating GRB2/ERK Signaling. J. Agric. Food Chem. 2018, 66, 10921–10929.
- Pattarayan, D.; Rajarajan, D.; Ayyanar, S.; Palanichamy, R.; Subbiah, R. C-phycocyanin suppresses transforming growth factor-β1-induced epithelial mesenchymal transition in human epithelial cells. Pharmacol. Reports 2017, 69, 426–431.
- Wen, R.; Sui, Z.; Zhang, X.; Zhang, S.; Qin, S. Expression of the phycoerythrin gene of Gracilaria lemaneiformis (Rhodophyta) in E. coli and evaluation of the bioactivity of recombinant PE. J. Ocean Univ. China 2007, 6, 373–377.
- Pan, Q.; Chen, M.; Li, J.; Wu, Y.; Zhen, C.; Liang, B. Antitumor function and mechanism of phycoerythrin from Porphyra haitanensis. Biol. Res. 2013, 46, 87–95.
- Cian, R.E.; López-Posadas, R.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Immunomodulatory properties of the protein fraction from Phorphyra columbina. J. Agric. Food Chem. 2012, 60, 8146–8154.
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222.
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406.
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J.; Jaouen, P.; Fleurence, J. Variation in the Biochemical Composition of the Edible Seaweed Grateloupia turuturu Yamada Harvested from Two Sampling Sites on the Brittany Coast (France): The Influence of Storage Method on the Extraction of the Seaweed Pigment R-Phycoerythrin. J. Chem. 2013, 2013, 1–8.
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 2011, 28, 45–49.
- Montoya, E.J.O.; Dorion, S.; Atehortua-Garcés, L.; Rivoal, J. Phycobilin heterologous production from the Rhodophyta Porphyridium cruentum. J. Biotechnol. 2021, 341, 30–42.
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785–792.
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Reliable quantification of R-phycoerythrin from red algal crude extracts. J. Appl. Phycol. 2020, 32, 1421–1428.
- Liu, S.; Chen, Y.; Lu, Y.; Chen, H.; Li, F.; Qin, S. Biosynthesis of fluorescent cyanobacterial allophycocyanin trimer in Escherichia coli. Photosynth. Res. 2010, 105, 135–142.
- Chen, H.; Lin, H.; Li, F.; Jiang, P.; Qin, S. Biosynthesis of a stable allophycocyanin beta subunit in metabolically engineered Escherichia coli. J. Biosci. Bioeng. 2013, 115, 485–489.
- Rossano, R.; Ungaro, N.; D’Ambrosio, A.; Liuzzi, G.M.; Riccio, P. Extracting and purifying R-phycoerythrin from Mediterranean red algae Corallina elongata Ellis & Solander. J. Biotechnol. 2003, 101, 289–293.
- Van Der Weij-De Wit, C.D.; Doust, A.B.; Van Stokkum, I.H.M.; Dekker, J.P.; Wilk, K.E.; Curmi, P.M.G.; Scholes, G.D.; Van Grondelle, R. How energy funnels from the phycoerythrin antenna complex to photosystem i and photosystem II in cryptophyte Rhodomonas CS24 cells. J. Phys. Chem. B 2006, 110, 25066–25073.
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1173–1193.
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112.
- Dumay, J.; Morançais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I.A., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 275–318.
- Brauch, J.E. Underutilized Fruits and Vegetables as Potential Novel Pigment Sources. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–335.
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266.
- Dagnino-Leone, J.; Figueroa, M.; Mella, C.; Vorphal, M.A.; Kerff, F.; Vásquez, A.J.; Bunster, M.; Martínez-Oyanedel, J. Structural models of the different trimers present in the core of phycobilisomes from Gracilaria chilensis based on crystal structures and sequences. PLoS ONE 2017, 12, e0177540.
- Liu, J.Y.; Zhang, J.P.; Wan, Z.L.; Liang, D.C.; Zhang, J.P.; Wu, H.J. Crystallization and preliminary X-ray studies of allophycocyanin from red alga Porphyra yezoensis. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 662–664.
- Liu, J.Y.; Jiang, T.; Zhang, J.P.; Liang, D.C. Crystal Structure of Allophycocyanin from Red Algae Porphyra yezoensis at 2.2-Å Resolution. J. Biol. Chem. 1999, 274, 16945–16952.
- Guo, Y.; Zang, X.; Cao, X.; Zhang, F.; Sun, D.; Shang, M.; Li, R.; Yangzong, Z.; Wei, X.; Zhang, X. Cloning and expression of Allophycocyanin gene from Gracilariopsis lemaneiformis and studying the binding sites of phycocyanobilin on its α and β subunits. J. Appl. Phycol. 2020, 32, 2657–2671.
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Extraction and quantification of phycobiliproteins from the red alga Furcellaria lumbricalis. Algal Res. 2019, 37, 115–123.
- Ismail, M.M.; Osman, M.E.H. Seasonal fluctuation of photosynthetic pigments of most common red seaweeds species collected from Abu Qir, Alexandria, Egypt. Rev. Biol. Mar. Oceanogr. 2016, 51, 515–525.
- Lüder, U.H.; Knoetzel, J.; Wiencke, C. Acclimation of photosynthesis and pigments to seasonally changing light conditions in the endemic antarctic red macroalga Palmaria decipiens. Polar Biol. 2001, 24, 231–236.
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid profiling of a red seaweed Pyropia yezoensis: Insights into biosynthetic pathways in the order Bangiales. Mar. Drugs 2018, 16, 426.
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic Aspects of Carotenoid Health Benefits-Where are we Now? Nutr. Res. Rev. 2021, 34, 276–302.
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of marine carotenoids. Mar. Drugs 2018, 16, 397.
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203.
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216.
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease—Current state of knowledge. J. Funct. Foods 2017, 38, 45–65.
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762.
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92.
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2020, 5, 1–51.
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386.
- Dias, M.G.; Borge, G.I.A.; Kljak, K.; Mandić, A.I.; Mapelli-Brahm, P.; Olmedilla-Alonso, B.; Pintea, A.M.; Ravasco, F.; Šaponjac, V.T.; Sereikaitė, J.; et al. European Database of Carotenoid Levels in Foods. Factors Affecting Carotenoid Content. Foods 2021, 10, 912.
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004.
- Aldred, E.M.; Buck, C.; Vall, K. Terpenes. In Pharmacology: A Handbook for Complementary Healthcare Professionals; Aldred, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; p. 362.
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396.
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182.
- Latowski, D.; Szymanska, R.; Strzalka, K. Carotenoids Involved in Antioxidant System of Chloroplasts. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 289–319.
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214.
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18.
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103.
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids—A review. RSC Adv. 2015, 5.
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321.
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96.
- King, J.W.; Srinivas, K.; Zhang, D. Advances in Critical Fluid Processing. In Alternatives to Conventional Food Processing; Proctor, A., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2010; pp. 93–144.
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; Mackenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008.
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251.
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta—Mol. Basis Dis. 2005, 1740, 108–115.
- Ravikrishnan, R.; Rusia, S.; Ilamurugan, G.; Salunkhe, U.; Deshpande, J.; Shankaranarayanan, J.; Shankaranarayana, M.L.; Soni, M.G. Safety assessment of lutein and zeaxanthin (LutemaxTM 2020): Subchronic toxicity and mutagenicity studies. Food Chem. Toxicol. 2011, 49, 2841–2848.
- Firdous, A.P.; Kuttan, G.; Kuttan, R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm. Biol. 2015, 53, 961–967.
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107.
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12.
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727.
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457.
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40.
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 101574.
- González, S.; Astner, S.; An, W.; Goukassian, D.; Pathak, M.A. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Investig. Dermatol. 2003, 121, 399–405.
- Kato, K.; Shinoda, T.; Nagao, R.; Akimoto, S.; Suzuki, T.; Dohmae, N.; Chen, M.; Allakhverdiev, S.I.; Shen, J.R.; Akita, F.; et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 2020, 11, 238.
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sensors Int. 2020, 1, 100058.
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188.
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891.
- Halim, R.; Hosikian, A.; Lim, S.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632.
- Lee, H.G.; Lu, Y.A.; Je, J.G.; Jayawardena, T.U.; Kang, M.C.; Lee, S.H.; Kim, T.H.; Lee, D.S.; Lee, J.M.; Yim, M.J.; et al. Effects of Ethanol Extracts from Grateloupia elliptica, a Red Seaweed, and Its Chlorophyll Derivative on 3T3-L1 Adipocytes: Suppression of Lipid Accumulation through Downregulation of Adipogenic Protein Expression. Mar. Drugs 2021, 19, 91.
- Chen, K.; Roca, M. Cooking effects on bioaccessibility of chlorophyll pigments of the main edible seaweeds. Food Chem. 2019, 295, 101–109.
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of chlorophyll a from biological soil crusts: A comparison of solvents for spectrophotometric determination. Soil Biol. Biochem. 2011, 43, 853–856.
- Samarasinghe, N.; Fernando, S.; Lacey, R.; Faulkner, W.B. Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew. Energy 2012, 48, 300–308.
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589.
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47.
- Milne, B.F.; Toker, Y.; Rubio, A.; Nielsen, S.B. Unraveling the intrinsic color of chlorophyll. Angew. Chemie—Int. Ed. 2015, 54, 2170–2173.
- Lumbessy, S.Y.; Junaidi, M.; Diniarti, N.; Setyowati, D.N.; Mukhlis, A.; Tambaru, R. Identification of chlorophyll pigment on Gracilaria salicornia seaweed. IOP Conf. Ser. Earth Environ. Sci. 2021, 681, 12017.
- Torres, P.B.; Chow, F.; Furlan, C.M.; Mandelli, F.; Mercadante, A.; dos Santos, D.Y.A.C. Standardization of a protocol to extract and analyze chlorophyll a and carotenoids in Gracilaria tenuistipitata var. liui. Zhang and Xia (Rhodophyta). Braz. J. Oceanogr. 2014, 62, 57–63.
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633.
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (I) Phytylated derivatives. J. Chromatogr. A 2015, 1406, 99–108.
- Dias, A.M.; Ferreira, M.L.S. “Supermarket Column Chromatography of Leaf Pigments” Revisited: Simple and Ecofriendly Separation of Plant Carotenoids, Chlorophylls, and Flavonoids from Green and Red Leaves. J. Chem. Educ. 2014, 92, 189–192.
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688.
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8.
- Spears, K. Developments in food colourings: The natural alternatives. Trends Biotechnol. 1988, 6, 283–288.
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their Derivatives Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1194–1222.
- ChemicalBook:Chlorophyll A. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB5471362_EN.htm (accessed on 22 November 2021).
- Janarthanan, M.; Senthil Kumar, M. The properties of bioactive substances obtained from seaweeds and their applications in textile industries. J. Ind. Text. 2017, 48, 361–401.
