Pheomelanin is a natural yellow-reddish sulfur-containing pigment derived from tyrosinase-catalyzed oxidation of tyrosine in presence of cysteine. It is one of the existing forms of the natural pigment melanin, which is present in the skin in two forms: eumelanin and pheomelanin. Generally, the formation of melanin pigments is a protective response against the damaging effects of UV radiation in skin.
- pheomelanin
- nitrotyrosine
- dityrosine
- photooxidation
1. Introduction
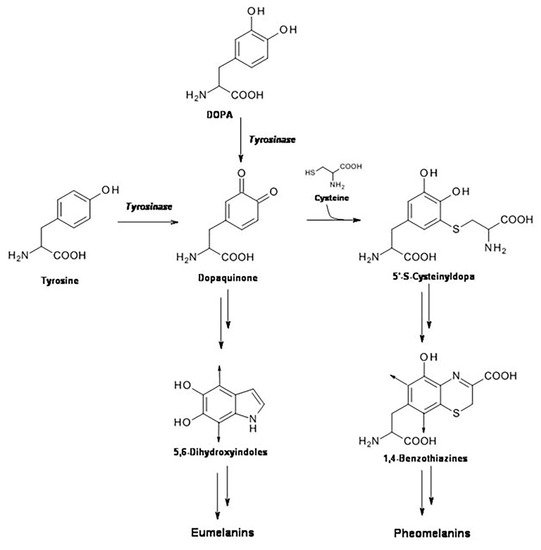
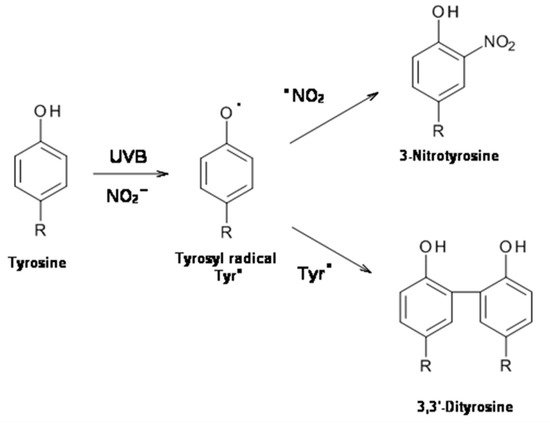
2. UVB Radiation-Induced Photooxidation/Photonitration of l-Tyrosine
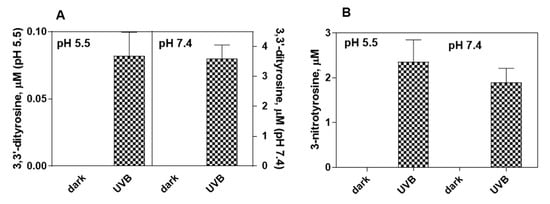
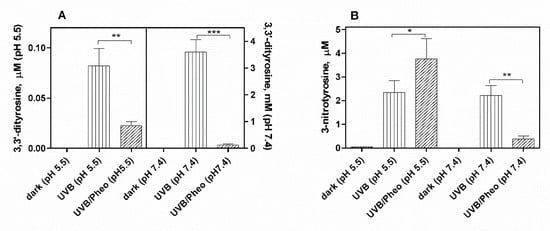
3. Effect of Pheomelanin on UVB Radiation-Induced Photooxidation/Photonitration of l-Tyrosine
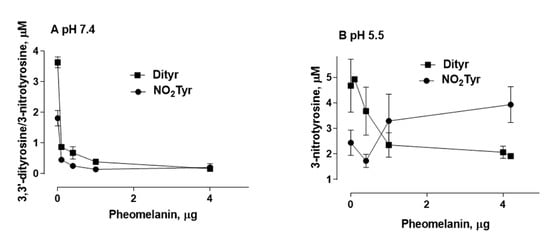
4. Photoproperties of Pheomelanin on UVB-Induced Oxidative/Nitrative Modifications of l-Tyrosine: Effect of Fe(III)
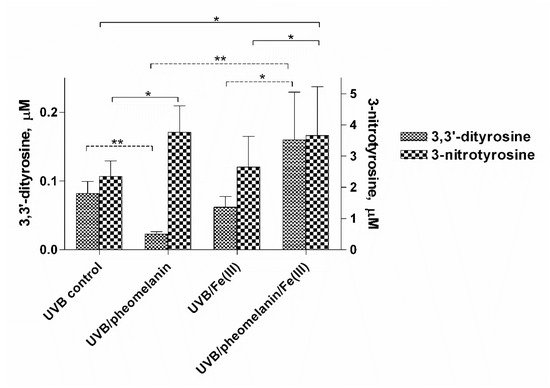
5. Pheomelanin Effect on Oxidative/Nitrative Modifications of l-Tyrosine Induced by UVB Radiation: Role of Singlet Oxygen
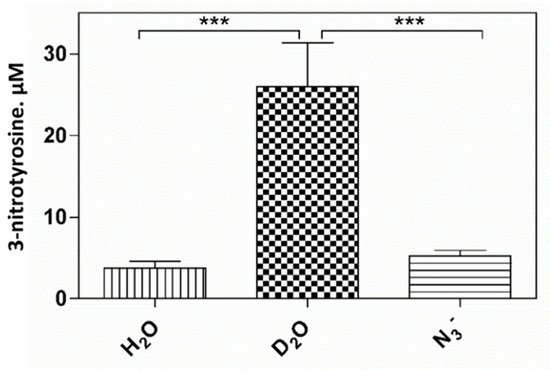
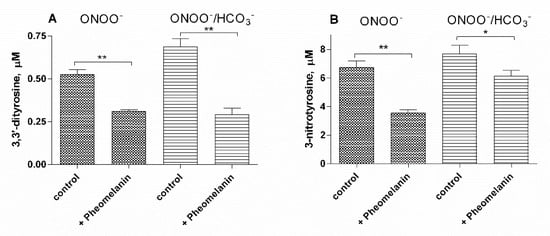
6. Pheomelanin Effect on Oxidative/Nitrative Modifications of l-Tyrosine Induced by Peroxyitrite
7. Discussion and Conclusions
The results of this study show that the photoproperties of pheomelanin can be modulated by various experimental conditions. These properties were studied by analyzing the effect of pheomelanin on UVB radiation-induced oxidation/nitration of tyrosine. UVB irradiation leads to the dimerization of tyrosine with the formation of 3,3′-dityrosine and in the presence of nitrite the photochemical reaction forms 3-nitrotyrosine as an additional product. In the presence of pheomelanin, tyrosine is dose-dependently protected from oxidation to 3,3′-dityrosine both at pH 5.5 and physiological pH (pH 7.4). Pheomelanin can perform a protective function on the conversion of tyrosine to 3-nitrotyrosine at pH 7.4. The experiments conducted on the formation of 3,3′-dityrosine and 3-nitrotyrosine induced by peroxynitrite (ONOO−) confirm this hypothesis. Peroxynitrite, which is generated in vivo from the reaction of nitric oxide (•NO) with the superoxide anion (O2•−), is a very reactive species capable of nitrating and oxidizing tyrosine. Pheomelanin showed protective properties both on the formation of 3,3′-dityrosine and on the conversion of tyrosine to 3-nitrotyrosine induced both by peroxynitrite and peroxynitrite-CO2 adduct. These results indicate that pheomelanin can act as free radical scavenger and the observed protective action of the pigment on UVB-induced tyrosine modifications can be attributed to this property.
An interesting result that emerged from our investigations is that pheomelanin can have pro-oxidant properties under some experimental conditions. We observed that the nitration of tyrosine to 3-nitrotyrosine induced by UVB radiation in presence of nitrite at pH 5.5 is increased when carried out in the presence of pheomelanin. These results indicate that the properties of pheomelanin can be significantly influenced by the pH during UVB irradiation, switching from antioxidant (pH 7.4) to pro-oxidant (pH 5.5). Furthermore, pheomelanin has a remarkable ability to bind metals and this property leads often to a modification of the photoprotective capabilities of the pigment. In our experimental conditions by adding Fe(III), we observed a reduced ability to inhibit the oxidative reaction. The pigment bond with iron induces an increase in the production of highly oxidizing reactive species whose action can only be partially counteracted by the antioxidant activity of the pigment itself. The investigations carried out to obtain information on the mechanism through which the nitrite/pheomelanin/UVB system induces the nitration of tyrosine at pH 5.5 indicate that, in our experimental conditions, the process can involve singlet oxygen, indeed, in the presence of D2O, the production of 3-nitrotyrosine is considerably higher than that formed in H2O.
The result presented herein indicates that photoproperties of pheomelanin can be modulated by various experimental conditions. It is well-known that pheomelanin undergoes structural modifications by UV rays. In the course of the biosynthetic pathways, modification involves benzothiazine units which are gradually converted to benzothiazole [4]. The relative ratio of these two types of pheomelanin moieties appears important in determining whether pheomelanin acts as a pro-oxidant [5]. Under our experimental conditions, UVB radiation and reactive nitrogen species could similarly influence pigment photoreactivity and induce structural modifications of pheomelanin worth to be further explored.
In conclusion pheomelanin is able to perform a protective function both on the tyrosine oxidation to 3,3′-dityrosine and on the conversion of tyrosine to 3-nitrotyrosine when the exposure is conducted at physiological pH; conversely at pH 5.5, the presence of pheomelanin induces a 60% increase in the formation of 3-nitrotyrosine. The addition of Fe(III) during the irradiation of tyrosine in presence of nitrite provokes a decrease of the antioxidant activity of pheomelanin also against the formation of 3,3’-dityrosine, so the photoproperties of pheomelanin may be affected by the presence of metal ions. Finally, pheomelanin showed protective properties on oxidation/nitration of tyrosine induced by peroxynitrite and by the decomposition of the peroxynitrite-CO2 adduct.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23010267
References
- Prota, G. The Chemistry of Melanins and Melanogenesis. Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 1995; Volume 64, pp. 93–148.
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579.
- Napolitano, A.; De Lucia, M.; Panzella, L.; D’Ischia, M. The “Benzothiazine” Chromophore of Pheomelanins: A Reassessment. Photochem. Photobiol. 2008, 84, 593–599.
- Wakamatsu, K.; Ohtara, K.; Ito, S. Chemical analysis of late stages of pheomelanogenesis: Conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res. 2009, 22, 474–486.
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; D’Ischia, M. “Fifty Shades” of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties. Int. J. Mol. Sci. 2016, 17, 746.
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin†. Photochem. Photobiol. 2008, 84, 539–549.
- Rouzaud, F.; Kadekaro, A.L.; Abdel-Malek, Z.A.; Hearing, V.J. MC1R and the response of melanocytes to ultraviolet radiation. Mutat. Res. Mol. Mech. Mutagenes. 2005, 571, 133–152.
- Chedekel, M.R.; Smith, S.K.; Post, P.W.; Pokora, A.; Vessell, D.L. Photodestruction of pheomelanin: Role of oxygen. Proc. Natl. Acad. Sci. USA 1978, 75, 5395–5399.
- Panzella, L.; Szewczyk, G.; D’Ischia, M.; Napolitano, A.; Sarna, T. Zinc-induced Structural Effects Enhance Oxygen Consumption and Superoxide Generation in Synthetic Pheomelanins on UVA/Visible Light Irradiation†. Photochem. Photobiol. 2010, 86, 757–764.
- Takeuchi, S.; Zhang, W.; Wakamatsu, K.; Ito, S.; Hearing, V.J.; Kraemer, K.H.; Brash, D.E. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc. Natl. Acad. Sci. USA 2004, 101, 15076–15081.
- Wenczl, E.; Van Der Schans, G.P.; Roza, L.; Kolb, R.M.; Timmerman, A.J.; Smit, N.P.M.; Pavel, S.; Schothorst, A.A. (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J. Investig. Dermatol. 1998, 111, 678–682.
- Sarna, T.; Menon, I.A.; Sealy, R.C. Photoinduced oxygen consumption in melanin systems—II. Action spectra and quantum yields for pheomelanins. Photochem. Photobiol. 1984, 39, 805–809.
- Ezzahir, A. The influence of melanins on the photoperoxidation of lipids. J. Photochem. Photobiol. B Biol. 1989, 3, 341–349.
- Menon, I.A.; Persad, S.; Haberman, H.F.; Kurian, C.J. A comparative study of the physical and chemical properties of melanins isolated from human black and red hair. J. Investig. Dermatol. 1983, 80, 202–206.
- Menon, I.A.; Persad, S.; Ranadive, N.S.; Haberman, H.F. Role of superoxide and hydrogen peroxide in cell lysis during irradiation in vitro of Ehrlich ascitic carcinoma cells in the presence of melanin. Can. J. Biochem. Cell Biol. 1985, 63, 278–283.
- Felix, C.C.; Hyde, J.S.; Sealy, R.C. Photoreactions of melanin: A new transient species and evidence for triplet state involvement. Biochem. Biophys. Res. Commun. 1979, 88, 456–461.
- Sarna, T.; Menon, I.A.; Sealy, R.C. Photosensitization of melanins: A comparative study. Photochem. Photobiol. 1985, 42, 529–532.
- Foote, C.S. Photosensitized Oxidation and Singlet Oxygen: Consequences in Biological Systems. In Free Radicals in Biology; Elsevier: Amsterdam, The Netherlands, 1976; pp. 85–133.
- Ambrosio, A.L.; Boyle, J.A.; Aradi, A.E.; Christian, K.A.; Di Pietro, S.M. TPC2 controls pigmentation by regulating melanosome pH and size. Proc. Natl. Acad. Sci. USA 2016, 113, 5622–5627.
- Wakamatsu, K.; Nagao, A.; Watanabe, M.; Nakao, K.; Ito, S. Pheomelanogenesis is promoted at a weakly acidic pH. Pigment Cell Melanoma Res. 2017, 30, 372–377.
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316.
- Cai, S.; Saito, T.; Fujii, N. Simultaneous ultraviolet B-induced photo-oxidation of tryptophan/tyrosine and racemization of neighboring aspartyl residues in peptides. Free Radic. Biol. Med. 2013, 65, 1037–1046.
- Giulivi, C.; Traaseth, N.J.; Davies, K.J.A. Tyrosine oxidation products: Analysis and biological relevance. Amino Acids 2003, 25, 227–232.
- Giulivi, C.; Davies, K.J. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J. Biol. Chem. 1993, 268, 8752–8759.
- Ischiropoulos, H. Biological Tyrosine Nitration: A Pathophysiological Function of Nitric Oxide and Reactive Oxygen Species. Arch. Biochem. Biophys. 1998, 356, 1–11.
- Van der Vliet, A.; Eiserich, J.P.; Halliwell, B.; Cross, C.E. Formation of Reactive Nitrogen Species during Peroxidase-catalyzed Oxidation of Nitrite. J. Biol. Chem. 1997, 272, 7617–7625.
- Sampson, J.B.; Ye, Y.; Rosen, H.; Beckman, J.S. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch. Biochem. Biophys. 1998, 356, 207–213.
- Brennan, M.L.; Wu, W.; Fu, X.; Shen, Z.; Song, W.; Frost, H.; Vadseth, C.; Narine, L.; Lenkiewicz, E.; Borchers, M.T.; et al. A tale of two controversies. Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002, 277, 17415–17427.
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; Van Der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397.
- Burner, U.; Furtmüller, P.G.; Kettle, A.J.; Koppenol, W.H.; Obinger, C. Mechanism of reaction of myeloperoxidase with nitrite. J. Biol. Chem. 2000, 275, 20597–20601.
- Gaut, J.P.; Byun, J.; Tran, H.D.; Lauber, W.M.; Carroll, J.A.; Hotchkiss, R.S.; Belaaouaj, A.; Heinecke, J.W. Myeloperoxidase produces nitrating oxidants in vivo. J. Clin. Investig. 2002, 109, 1311–1319.
- Capuozzo, E.; Pecci, L.; Giovannitti, F.; Baseggio Conrado, A.; Fontana, M. Oxidative and nitrative modifications of enkephalins by human neutrophils: Effect of nitroenkephalin on leukocyte functional responses. Amino Acids 2012, 43, 875–884.
- Pecci, L.; Montefoschi, G.; Antonucci, A.; Costa, M.; Fontana, M.; Cavallini, D. Formation of nitrotyrosine by methylene blue photosensitized oxidation of tyrosine in the presence of nitrite. Biochem. Biophys. Res. Commun. 2001, 289, 305–309.
- Fontana, M.; Blarzino, C.; Pecci, L. Formation of 3-nitrotyrosine by riboflavin photosensitized oxidation of tyrosine in the presence of nitrite. Amino Acids 2012, 42, 1857–1865.
- Silva, E.; Godoy, J. Riboflavin sensitized photooxidation of tyrosine. Int. J. Vitam. Nutr. Res. 1994, 64, 253–256.
- Lu, C.Y.; Liu, Y.Y. Electron transfer oxidation of tryptophan and tyrosine by triplet states and oxidized radicals of flavin sensitizers: A laser flash photolysis study. Biochim. Biophys. Acta Gen. Subj. 2002, 1571, 71–76.
- Schopfer, F. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003, 28, 646–654.
- Gow, A.J.; Farkouh, C.R.; Munson, D.A.; Posencheg, M.A.; Ischiropoulos, H. Biological significance of nitric oxide-mediated protein modifications. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L262–L268.
- Atwood, C.S.; Perry, G.; Zeng, H.; Kato, Y.; Jones, W.D.; Ling, K.Q.; Huang, X.; Moir, R.D.; Wang, D.; Sayre, L.M.; et al. Copper Mediates Dityrosine Cross-Linking of Alzheimer’s Amyloid-β. Biochemistry 2004, 43, 560–568.
- Monteiro, H.P. Signal transduction by protein tyrosine nitration: Competition or cooperation with tyrosine phosphorylation-dependent signaling events? Free Radic. Biol. Med. 2002, 33, 765–773.
- Park, J.Y.; Lee, Y.N. Solubility and decomposition kinetics of nitrous acid in aqueous solution. J. Phys. Chem. 1988, 92, 6294–6302.
- Schmitz, S.; Thomas, P.D.; Allen, T.M.; Poznansky, M.J.; Jimbow, K. Dual role of melanins and melanin precursors as photoprotective and phototoxic agents: Inhibition of ultraviolet radiation-induced lipid peroxidation. Photochem. Photobiol. 1995, 61, 650–655.
- Sealy, R.C.; Felix, C.C.; Hyde, J.S.; Swartz, H.M. Structure and Reactivity of Melanins: Influence of Free Radicals and Metal Ions. In Free Radicals in Biology; Elsevier: Amsterdam, The Netherlands, 1980; pp. 209–259.
- Palumbo, A.; D’Ischia, M.; Misuraca, G.; Prota, G.; Schultz, T.M. Structural modifications in biosynthetic melanins induced by metal ions. Biochim. Biophys. Acta Gen. Subj. 1988, 964, 193–199.
- Zadlo, A.; Mokrzyński, K.; Ito, S.; Wakamatsu, K.; Sarna, T. The influence of iron on selected properties of synthetic pheomelanin. Cell Biochem. Biophys. 2020, 78, 181–189.
- Rodgers, M.A.J.; Snowden, P.T. Lifetime of oxygen (O2(1.DELTA.g)) in liquid water as determined by time-resolved infrared luminescence measurements. J. Am. Chem. Soc. 1982, 104, 5541–5543.
- Lymar, S.V.; Jiang, Q.; Hurst, J.K. Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochemistry 1996, 35, 7855–7861.
- Goldstein, S.; Czapski, G.; Lind, J.; Merényi, G. Tyrosine nitration by simultaneous generation of NO and O2/-under physiological conditions. How the radicals do the job. J. Biol. Chem. 2000, 275, 3031–3036.
- Napolitano, A.; Panzella, L.; Monfrecola, G.; D’Ischia, M. . Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma.; Pigment Cell Melanoma Res.: Italy , 2014; pp. 721–733.
