Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Environmental
Microbial fuel cells (MFC) are an emerging technology for wastewater treatment that utilizes the metabolism of microorganisms to generate electricity from the organic matter present in water directly.
- microbial fuel cell
- ion-exchange membrane
- non-fluorinated membrane
1. Introduction
Many wastewater treatment techniques are used to remove contaminants from wastewater so that it can be returned safely to the water cycle. Typically, these include anaerobic digestion, aerobic activated sludge process, chemical precipitation, coagulation, flocculation, neutralization, adsorption, etc. These technologies require energy for operating and dealing with sludge management issues. Anaerobic digestion also generates sewage/biogas while effectively treating the sewage. Biogas is a mixture of methane (CH4), carbon dioxide (CO2), and water (H2O). The process of generating methane is called methanogenesis, and the microorganism involved in the process are methanogens. Methane can be used as fuel for heating, transport or generating electricity.
On the other hand, in a microbial fuel cell (MFC), the electroactive microorganism called exoelectrogen consumes organic matter present in wastewater and converts it directly into electricity. The microorganism oxidizes the organic matter and produces H+ ions (protons) and electrons in the anodic chamber. Protons are allowed to migrate towards the cathode chamber through the proton exchange membrane, and the electrons are made to flow via an external circuit to the cathode, where the reduction of H+ ion takes place.
Barnett Cohen observed this phenomenon in 1931 and created microbial half fuel cells (MFC) that, when connected in series, were capable of producing over 35 volts with only a current of 2 milliamps [1]. Over the years, it has been shown that MFC technology has potential for wastewater treatment and energy generation. There are other types of devices that generate electricity directly from fuel, such as polymer electrolyte membrane fuel cell (PEM), reverse polymer electrolyte membrane fuel cell (RePEM) and store energy such as redox flow batteries (RFB) which requires heavy metals [2][3]. The unique capability of MFC is to treat wastewater and produce electricity from microbial activity. Microorganisms not only reduced organic matter in the wastewater, but recent studies have found metal-reducing microorganisms that can also treat wastewater containing heavy and toxic metals [4][5]. PEM fuel cell requires an external source of hydrogen and oxygen gases to run the system [6]. In comparison, MFC is eco-friendly and self-sustainable technology to produce energy from waste material.
There are several papers in the last few decades that investigated MFCs for removal of BOD and COD [7], removal of toxic and heavy metals [8], and of course, generating electricity simultaneously [9]. Other applications include biosensors [10][11], bioremediations [12], nitrification and denitrification [13]. Some researchers also demonstrate the removal of various salts from saline water [14][15]. By modifying MFC’s architecture and operating conditions, the technology can be used to produce hydrogen. It is claimed that less energy is required compared to the electrolysis of H2O to produce hydrogen [16]. The researchers showed that carbon dioxide could be reduced in the anodic chamber to form methane gas [17]. However, many of these findings are preliminary studies at the laboratory level. In order to develop an application based MFC system such as removing heavy metals from wastewater, the design and operating conditions of the MFC must be enhanced [18]. The architecture and materials of the key components such as electrodes and membranes are critical for the optimal performance of the MFC [19].
2. Working Principle and Components of MFC
The schematic of MFC is shown in (Figure 1). The wastewater is fed into the anode chamber, where active microbes form a biofilm on the anode surface and microbes oxidize organic matter to produce electrons that are made to flow through an external circuit to reach the cathode. While the protons (H+) move internally through the proton exchange membrane towards the cathode chamber and meet electrons to complete the reaction by producing pure water [20]. The purpose of using a proton exchange membrane in between anodic and cathodic chambers is to allow only the H+ (proton) ions produced in the anodic chamber to pass through towards the cathodic chamber internally [21][22]. Electricity is generated due to the flow of electrons through the external circuit. There are two different reactions at anode and cathode; each has a different reaction rate and kinetics [23]. Each of these components contributes to the device’s performance. Hence, it is crucial to characterize the components to improve the performance of MFC.
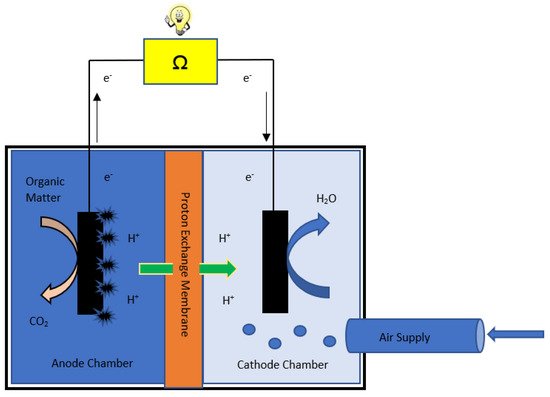
Figure 1. A schematic diagram of a microbial fuel cell (MFC).
Considering glucose as an example substrate [24][25], typical anodic and the cathodic half-reactions are given below [26]:
Anode half-reaction:
C6H12O6 + 6H2O → 6CO2 +24 H+ + 24e−
Cathode half reaction:
6O2 + 24e− + 24H+ → 12H2O
Complete reaction:
C6H12O6 + 6O2→ 6CO2 + 6H2O
Open circuit voltage generated can be calculated from the Gibbs free energy of water formation and charge of the electrons [27].
where n is the number of exchanged electrons and F is Faraday’s constant. For the oxidation of hydrogen and water formation, n = 2; Gibbs free energy, ΔG is −237,130 J/mol; F = Faraday’s constant value is 96,480 C/mol [28]. Therefore, for generating energy for useful purposes, a number of cells need to be stacked [22].
ΔE = −ΔG/nF
ΔE = 237,130 (J/mol)/2 × 96,480 (C/mol) = 1.23 Volts
3. Role of Proton Exchange Membrane and Characterization
The performance of an MFC depends on the microbial activity in the anodic chamber and the efficient passage of the protons into the cathodic chamber. In this regard, the membrane acts as a boundary between the anaerobic anode chamber and aerobic cathode and no undesired mixing of species between chambers is allowed [29]. The membrane should also facilitate transportation of only H+ ion from anode to cathode and repel all other anions and negatively charged particles and prevent crossover of O2 from cathodic to the anodic chamber.
Proton exchange membranes are partially permeable and made of ionomers that only conducts protons and restricts electrons. The membrane also does not permit the flow of any gaseous products (i.e., carbon dioxide (CO2) in the anodic chamber and oxygen (O2) in the cathodic chamber) [30]. The most commonly used membrane is Nafion from DuPont, an ionomer with a perfluorinated backbone such as Teflon [26]. Nafion has been widely used in other types of fuel cells and batteries such as polymer electrolyte membrane fuel cells (PEMFC), vanadium redox flow batteries (VRFBs) etc. [31]. The conductive co-polymer Nafion is a sulfonated tetrafluoroethylene-based fluoropolymer [32]. Nafion has a high hydrophobicity due to the presence of perfluoroalkyl backbones. As a cation exchange polymer, Nafion prevents anionic species from reaching the electrode surface while allowing cation to pass through. The hydrophilic Nafion film has a negatively charged sulfonate group that provides selective preconcentration of positively charged particles via electrostatic interaction. In contrast, the hydrophobic fluorocarbon chain gives a selectivity for the hydrophobic part of the molecule [27]. Moreover, Nafion provides good chemical and mechanical stability due to the Polytetrafluoroethylene (PTFE) backbone and strong C-F bonds. The advantage of using Nafion as a membrane in MFC is its excellent proton conductivity due to the significant phase separation between hydrophilic and hydrophobic domains. Other advantages are good antifouling capacity and chemical compatibility [31].
Proton conductivity acts as the fundamental property of membranes when assessing the potential material for membranes, especially for electricity generation applications. Resistive losses are dependent on the proton conductivity and ionic resistance of the membrane. Research work has been done to analyze the effect of the thickness of the membrane on the output performance of MFC. The experiments were done for three different thicknesses 2.5, 5, and 10 mm of the membrane made of fine fire clay material. The maximum absolute power around 2.1 mW was obtained using a 2.5 mm thick membrane since a thinner membrane has lower ionic resistance than the thick one [33]. A research study was carried out to analyze the effect of the thickness of the Nafion membrane used in an iron-chromium redox flow battery with thicknesses of 50, 126, and 178 µm. The maximum utilization of electrolytes was observed with a thickness of 126 µm. The outcome of the research work implies that the thinner membrane has less internal resistance and higher permeability to electro active species [34]. Another research work was carried out to analyze the effect of the thickness of nanocomposite membrane on the output of MFC by using STAT 15 optimization tool. The experiments were carried out using three different thicknesses of the nanocomposite membrane, such as 100, 120, and 140 µm. The maximum power density of around 147 mW/m2 was obtained for the membrane thickness of 120 µm (0.12 mm) [35]. Although the thinnest membrane was 100 µm but this study indicates the need to optimize the thickness of the membrane to minimize the internal resistance, ionic loss and maximize the permeability of electroactive species. At a molecular level, the proton transport in hydrated polymeric matrices is based on two principal mechanisms: proton hopping technique in which protons are transferred from one site to another through the formation [36] and breaking of hydrogen bonds known as Grotthuss-type mechanism and diffusion mechanism where water acts as the vehicle [37][38].
This entry is adapted from the peer-reviewed paper 10.3390/en15020444
References
- Cohen, B. The Bacterial Culture as an Electrical Half-Cell. J. Bacteriol. 1931, 21, 18–19.
- Perna, A.; Kim, S. Pem Fuel Cells and Vanadium Redox Flow Batteries: Two. In Proceedings of the European Fuel Cell Technology & Applications Conference—Piero Lunghi Conference 2017, Naples, Italy, 12–15 December 2017; pp. 15–17.
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445.
- Gangadharan, P.; Nambi, I.M. Hexavalent chromium reduction and energy recovery by using dual-chambered microbial fuel cell. Water Sci. Technol. 2015, 71, 353–358.
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152.
- Zhang, H.; Shen, P.K. Recent Development of Polymer Electrolyte Membranes for Fuel Cells. Chem. Rev. 2012, 112, 2780–2832.
- Kaewkannetra, P.; Chiwes, W.; Chiu, T.Y. Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel 2011, 90, 2746–2750.
- Song, T.-S.; Jin, Y.; Bao, J.; Kang, D.; Xie, J. Graphene/biofilm composites for enhancement of hexavalent chromium reduction and electricity production in a biocathode microbial fuel cell. J. Hazard. Mater. 2016, 317, 73–80.
- Abbasi, U.; Jin, W.; Pervez, A.; Bhatti, Z.A.; Tariq, M.; Shaheen, S.; Iqbal, A.; Mahmood, Q. Anaerobic microbial fuel cell treating combined industrial wastewater: Correlation of electricity generation with pollutants. Bioresour. Technol. 2016, 200, 1–7.
- Yu, D.; Bai, L.; Zhai, J.; Wang, Y.; Dong, S. Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta 2017, 168, 210–216.
- Mukherjee, S.; Su, S.; Panmanee, W.; Irvin, R.T.; Hassett, D.J.; Choi, S. A microliter-scale microbial fuel cell array for bacterial electrogenic screening. Sens. Actuators A: Phys. 2013, 201, 532–537.
- He, Y.-R.; Xiao, X.; Li, W.-W.; Cai, P.-J.; Yuan, S.-J.; Yan, F.-F.; He, M.-X.; Sheng, G.-P.; Tong, Z.-H.; Yu, H.-Q. Electricity generation from dissolved organic matter in polluted lake water using a microbial fuel cell (MFC). Biochem. Eng. J. 2013, 71, 57–61.
- Feng, C.; Huang, L.; Yu, H.; Yi, X.; Wei, C. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170.
- Sevda, S.; Abu-Reesh, I.M.; Yuan, H.; He, Z. Bioelectricity generation from treatment of petroleum refinery wastewater with simultaneous seawater desalination in microbial desalination cells. Energy Convers. Manag. 2017, 141, 101–107.
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Bio-electrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J. Hazard. Mater. 2010, 177, 487–494.
- Venkata Mohan, S.; Mohanakrishna, G.; Srikanth, S.; Sarma, P. Harnessing of bioelectricity in microbial fuel cell (MFC) employing aerated cathode through anaerobic treatment of chemical wastewater using selectively enriched hydrogen producing mixed consortia. Fuel 2008, 87, 2667–2676.
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.; Park, W.; Kim, C.-W.; Kim, I.S. Methanogenesis control by employing various environmental stress conditions in two-chambered microbial fuel cells. Bioresour. Technol. 2010, 101, 5350–5357.
- Ezziat, L.; ElAbed, A.; Ibnsouda, S.; El Abed, S.; ElAbed, A. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal From Wastewater. Front. Energy Res. 2019, 7, 1–13.
- Mathuriya, A.S.; Jadhav, D.A.; Ghangrekar, M.M. Architectural adaptations of microbial fuel cells. Appl. Microbiol. Biotechnol. 2018, 102, 9419–9432.
- Kalia, V.C.; Kumar, P. Microbial Applications vol. 1 Bioremediation and Bioenergy; Springer: Cham, Switzerland, 2018.
- Das, D. Microbial Fuel Cell; Springer International Publishing: Cham, Switzerland, 2018.
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390.
- Oliveira, V.; Simões, M.; Melo, L.; Pinto, A. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64.
- Gezginci, M.; Uysal, Y. Electricity generation using different substrates and their different concentrations in microbial fuel cell. J. Environ. Prot. Ecol. 2014, 15, 1744–1750.
- Jafary, T.; Ghoreyshi, A.A.; Najafpour, G.D. The Effect of Substrate Concentration on the Electrical Performance of Microbial Fuel Cell (MFC). In Proceedings of the International Conference on Environment 2010, Penag, Malaysia, 13–15 December 2010.
- Rahimnejad, M.; Bakeri, G.; Najafpour, G.; Ghasemi, M.; Oh, S.-E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 2014, 1, 7–15.
- Gautam, D.; Anjum, S.; Ikram, S. Proton Exchange Membrane (PEM) in Fuel Cells: A Review. IUP J. Chem. 2010, III, 51–81.
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192.
- Dharmalingam, S.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2018.
- Ramirez-nava, J.; Mart, M.; Hern, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738.
- Sun, C.; Złotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. hybrid membranes for vanadium redox flow batteries. Solid State Ionics 2018, 319, 110–116.
- Mokhtarian, N.; Ghasemi, M.; Daud, W.R.W.; Ismail, M.; Najafpour, G.; Alam, J. Improvement of Microbial Fuel Cell Performance by Using Nafion Polyaniline Composite Membranes as a Separator. J. Fuel Cell Sci. Technol. 2013, 10, 041008.
- Merino-Jimenez, I.; Obata, O.; Pasternak, G.; Gajda, I.; Greenman, J.; Ieropoulos, I. Effect of microbial fuel cell operation time on the disinfection efficacy of electrochemically synthesised catholyte from urine. Process. Biochem. 2020, 101, 294–303.
- Sun, C.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019.
- Sugumar, M.; Kugarajah, V.; Dharmalingam, S. Optimization of operational factors using statistical design and analysis of nanofiller incorporated polymer electrolyte membrane towards performance enhancement of microbial fuel cell. Process. Saf. Environ. Prot. 2021.
- Ghosh, P.; Ramachandran, M.; Kumar, V. Review on Proton Exchange Membranes and its Application in Microbial Fuel Cells. Int. J. Mech. Eng. Technol. 2018, 9, 1632–1641. Available online: http://www.iaeme.com/IJMET/index.asp1632; http://www.iaeme.com/ijmet/issues.asp?JType=IJMET&VType=09&IType=13; http://www.iaeme.com/IJMET/issues; http://www.iaeme.com/IJMET/index.asp1633 (accessed on 21 December 2021).
- Breslau, B.R.; Miller, I.F. A hydrodynamic model for electroosmosis. Ind. Eng. Chem. Fundam. 1971, 10, 554–565.
- De Grotthuss, C.J.T. Sur la décomposition de l’eau et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique. Ann. Chim. 1806, 58, 54–73.
- Miskan, M.; Ismail, M.; Ghasemi, M.; Jahim, J.M.; Nordin, D.; Abu Bakar, M.H. Characterization of membrane biofouling and its effect on the performance of microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 543–552.
- Xu, J.; Sheng, G.-P.; Luo, H.-W.; Li, W.-W.; Wang, L.-F.; Yu, H.-Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824.
This entry is offline, you can click here to edit this entry!
