Emerging SARS-CoV-2 variants pose threats to vaccination campaigns against COVID-19. Being more transmissible than the original virus, the SARS-CoV-2 B.1.617 lineage, named the Delta variant, swept through the world in 2021. The mutations in the Delta’s spike protein shift the protein towards a net positive electrostatic potential. Compared to the wild-type spike, the Delta one shows a higher affinity towards heparan sulfate proteoglycans than ACE2. Cellular studies showed that syndecan-4, the syndecan isoform abundant in the lung, enhances the transmission of the Delta variant by attaching its mutated spike glycoprotein and facilitating its cellular entry. In addition to the attachment to the polyanionic heparan sulfate chains, the Delta spike’s molecular interactions with syndecan-4 also involve syndecan-4’s cell-binding domain that mediates cell-to-cell adhesion. Exogenously added heparin or syndecan-4 knockdown efficiently blocks the Delta variant’s cellular entry. A profound understanding of syndecan-4-mediated endocytosis enables the development of molecularly targeted yet simple strategies to reduce the Delta variant’s spread.
1. Introduction
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) took the world by storm [
1]. Unlike any other pandemic of modern times, SARS-CoV-2 and the related coronavirus disease 2019 (COVID-19) caused widespread panic in societies [
2]. Various non-pharmaceutical interventions (NPIs) were utilized worldwide to prevent viral spread. Lockdowns, school closures, and travel bans were among the many NPIs adopted, all exerting limited effect on SARS-CoV-2 transmission [
3,
4]. The introduction of new and highly efficient vaccines against COVID-19, on the other hand, reduced the prevalence of severe COVID-19 cases worldwide [
5]. However, the emergence of new variants poses a threat to the seemingly successful and efficient mass vaccination efforts against COVID-19 [
6]. Named as a “Variant of Concern” by the WHO, Delta is a highly contagious SARS-CoV-2 mutant, first identified in India last year [
7]. The SARS-CoV-2 Delta variant, also known as lineage B.1.617.2, or the Indian variant, swept rapidly through India and the United Kingdom, then reaching the United States, thus becoming the predominant variant worldwide (and recently being taken over by the emerging Omicron variant, now accounting for the majority of COVID-19 cases) [
8,
9]. The Delta variant has several mutations in its spike protein, leading to increased transmissibility and the ability to escape neutralizing antibodies triggered by the original SARS-CoV-2 strain [
10,
11,
12]. The Delta’s mutations result in multiple replacements of neutral or negatively charged amino acids with positively charged ones [
13]. On the other hand, the spike protein’s shift towards a positive electrostatic potential does not lead to increased affinity towards ACE2, a cell surface protease widely recognized as the main cell entry receptor for SARS-CoV-2 [
14,
15,
16,
17]. A recent study showed that mutating the intracellular domain of ACE2 does not affect the intracellular entry of SARS-CoV-2, suggesting that the internalization of the virus is driven by ACE2 independent receptors [
18]. According to these findings, the virus attaches to ACE2; however, the membrane engulfment and subsequent internalization is mediated by other, ACE2-independent endocytosis receptors.
2. Effect of ACE2 Overexpression on the Cellular Internalization of the Spike Proteins
The cell surface ACE2 has been widely recognized as the primary cell entry receptor for SARS-CoV-2 [
16,
17]. Possessing an ACE2 specific receptor-binding domain (RBD), the spike protein plays a crucial role in initiating the attachment to the ACE2 receptor [
16]. To study the involvement of ACE2 in the cellular entry of the wild-type (WT) SARS-CoV-2 spike and its Delta variant, ACE2 overexpressing 293T-ACE2 cells, along with standard 293T cells were treated with the WT spike protein and its Delta variant for 4 h at 37 °C at a concentration of 50 nM. Cellular uptake was then detected by incubating the spike-treated, fixed and permeabilized cells with Alexa Fluor 647 (AF 647) labeled antibody specific for the N-terminal His-tag of the recombinant WT and Delta spike proteins. Before the imaging flow cytometry analyses, surface-attached spikes were removed with trypsinization, according to the method described by Nakase et al. [
29,
30]. Hence, the imaging flow cytometer (Amnis FlowSight) only measured the internalized spike proteins [
25]. Imaging flow cytometry analyses revealed that the Delta spike is internalized by 293T cells more efficiently than the WT spike protein (
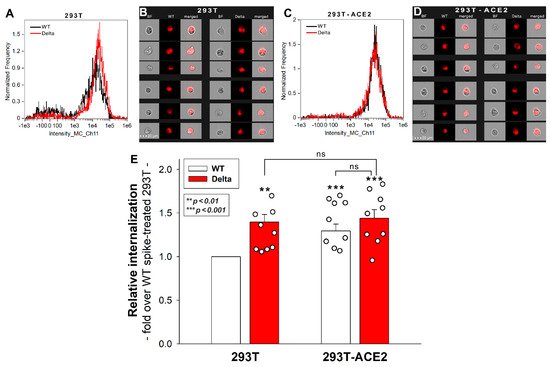
Figure 1A,B,E). Overexpression of ACE2 in 293T-ACE2 cells increased the internalization of the WT spike. Compared to Delta-treated 293T cells, Delta uptake into 293T-ACE cells remained almost unchanged, suggesting that Delta’s cellular entry is less dependent on ACE2 than WT spike’s (
Figure 1E). Incubating the cells with the fluorescently labeled anti-His tag antibodies without spike pretreatment did not induce any difference, showing that no specific binding influenced the obtained results. Additionally, neither spike protein affected cellular viability at the applied concentration of 50 nM.
Figure 1. Cellular uptake of WT and Delta spike into 293T and ACE2 overexpressing 293T-ACE2 cells. The cells were incubated with WT and Delta spike proteins for 4 h at 37 °C. After incubation, the cells were washed, trypsinized, fixed, permeabilized and treated with AF 647-labeled antibodies specific for the His-tag of the recombinant spike proteins. Cellular uptake was then analyzed with imaging flow cytometry. (A–D) Representative flow cytometry histograms (A,C), brightfield (BF) and fluorescent cellular images (B,D) showing the intracellular fluorescence of 293T and 293T-ACE2 cells treated with WT or Delta spike proteins. Scale bar = 20 μm. (E) Detected fluorescence intensities were normalized to WT spike-treated 293T cells as standards. The bars represent the mean + SEM of nine independent experiments. Experimental data are presented as dots. Statistical significance was assessed with ANOVA. ** p < 0.05 vs. standards; *** p < 0.01 vs. standards; ns: not significant.
3. 293T-ACE2 Cells Exhibit Increased ACE2 Yet Reduced HS and SDC Expression
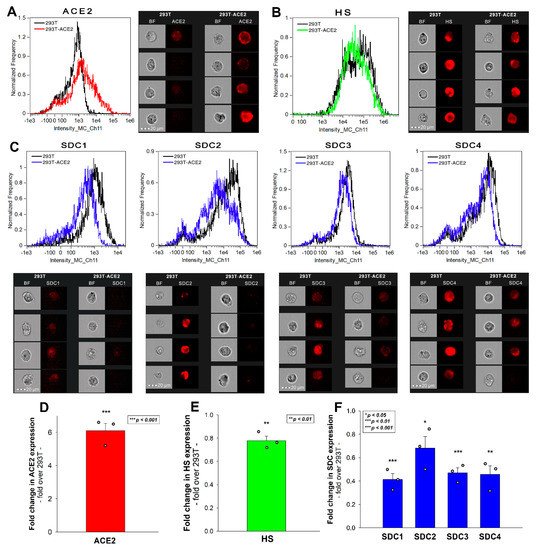
Simultaneously to the spike uptake studies, we also measured the expression of ACE2 and HS in both 293T and 293T-ACE2 cells. Compared to 239T, increased ACE2 expression of 293T-ACE2 cells (i.e., ACE2 expression was about six times that of 293T cells) was associated with significantly lower (~80%) cell surface HS expression (Figure 2A,B,D,E). To examine whether the expression of SDCs was also affected by ACE2 overexpression, we also measured SDC expression with flow cytometry. These analyses revealed significantly decreased the expression of all four SDC isoforms (i.e., SDC1–4) in ACE2 overexpressing ACE2-293T cells (Figure 2C,F). Studies with respective isotype controls revealed no difference in isotype control-treated 293T and 293T-ACE2 cells. Although the detailed molecular mechanism of ACE2 affecting membrane HS and SDC expression is still not fully understood (the lipid raft-associated ACE2 might disrupt the expression of lipid raft-associated HSPGs, including SDCs), this serendipity led to a valuable finding. In the case of the Delta spike, overexpression of ACE2 cannot compensate for the reduced HS and SDC content of 293T-ACE2 cells, highlighting the paramount importance of SDCs and polyanionic cell surface HSPGs in the interaction with the Delta spike, enriched in positively charged amino acids. Thus, the Delta spike protein exhibits a higher affinity towards HSPGs’ HS chains than towards ACE2.
Figure 2. ACE2, HS, and SDC expression of 293T and 293T-ACE2 cells. ACE2, HS, and SDC expression of 293T and 293T-ACE2 cells was measured with imaging flow cytometry by using fluorescently labeled specific antibodies. (A,B) ACE2 and HS expression profile of 293T and 293T-ACE2 cells. The representative flow cytometry histograms and cellular images show the ACE2 and HS expression of 293T and 293T-ACE2 cells treated with the fluorescent ACE2 and HS antibodies. Scale bar = 20 μm. (C) SDC expression profile of 293T and 293T-ACE2 cells. The representative flow cytometry histograms and fluorescent cellular images show the SDC expression of 293T cells and 293T-ACE2 cells treated with the APC-labeled respective SDC antibodies. Scale bar = 20 μm. (D–F) Detected ACE2, HS and SDC expression measures were normalized to those of 293T cells as standards. The bars represent the mean ± SEM of three independent experiments (data are represented as dots). Statistical significance vs. standards was assessed with ANOVA. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Effect of ACE2 and Proteoglycan Inhibition on Cellular Uptake
SARS-CoV-2 infection is initiated by the virus’ attachment to the host cells’ ACE2, while cellular entry is mediated by the host cell’s endocytic pathways [
16,
18]. Endocytosis is an active process orchestrated by signaling pathways stimulated by the ligand’s extracellular attachment to specific endocytic receptors [
31]. A recent cellular study showed that the ACE2-mediated signaling is not essential for SARS-CoV-2’s cellular entry, suggesting that endocytosis might be driven via ACE2-independent endocytic receptors [
18]. DX600 is a competitive peptide inhibitor of ACE2 that only modestly inhibits the cellular entry of SARS-CoV-2 (and -1), suggesting that ACE2’s enzymatic activity is not essential for viral endocytosis [
25,
32,
33]. On the other hand, heparin reduces SARS-CoV-1 and -2 infection very efficiently by directly competing with viral binding to host HSPGs [
22,
34,
35].
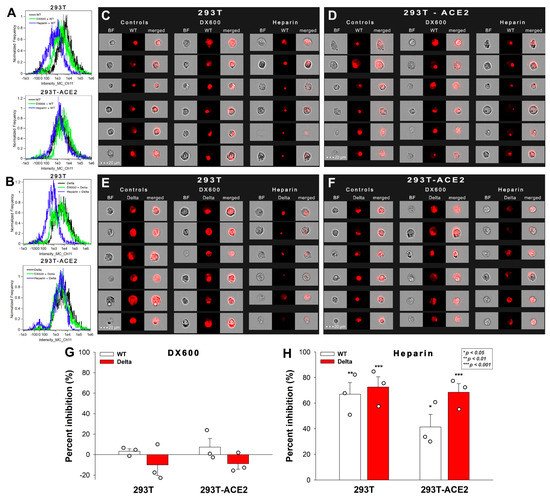
To further explore the role of ACE2 and HSPGs in the cellular uptake of the spike proteins, we studied the effect of the two competitive inhibitors of ACE2 and HSPGs (i.e., DX600 and heparin) on the cellular uptake of WT and Delta spike. Thus, 293T-ACE2 and standard 293T cells, preincubated with or without DX600 or heparin, were treated with the WT spike protein and its Delta variant for 4 h at 37 °C at a concentration of 50 nM. Unlike DX600, which left the cellular uptake of the spike proteins unaffected, heparin reduced the WT and Delta spike uptake in 293T and 293T-ACE2 cells, as shown by data obtained with imaging flow cytometry (Figure 3A–H). Heparin’s effect on lowering the cellular uptake of Delta was slightly higher than on WT spike, suggesting the increased importance of polyanionic HS chains in the interactions with the more positively charged Delta spike (Figure 3A–F,H). On the other hand, heparin’s effect on reducing the cellular uptake of WT spike into 293T-ACE2 cells was smaller than on 293T, showing that increased ACE2 expression leads to increased ACE2-dependent interactions with WT spike.
Figure 3. Effect of ACE2 and proteoglycan inhibition on cellular uptake. 293T and 293T-ACE2 cells, preincubated with or without heparin (200 ug/mL) or DX600 (10 uM), were treated with the WT or Delta spike proteins. (A–F) Representative flow cytometry histograms (A,B) and fluorescent cellular images (C–F) show the fluorescence of 293T and 293T-ACE2 cells treated with either the WT or Delta spike proteins in the presence or absence of the inhibitors. Scale bar = 20 μm. (G,H) The effect of the inhibitors expressed as percent inhibition, calculated with the following formula: ((X − Y)/X) × 100, where X is the fluorescence intensity obtained on cells treated with the spike proteins in the absence of the inhibitor, and Y is the fluorescence intensity obtained on cells treated with proteins in the presence of the inhibitor. The bars represent the mean ± SEM of three independent experiments (experimental data are represented as dots). Statistical significance vs. standards was assessed with ANOVA. * p < 0.05; ** p < 0.01; *** p < 0.001.
These results also showed that, compared to WT spike, Delta has a greater affinity towards HSPGs, than towards ACE2. The observation that ACE2 activity is not required for the cellular entry of either the WT or the Delta spike proteins suggests that ACE2 functions as a binding site instead of a receptor actively driving the mechanism of viral endocytosis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020796