Cyanobacteria, also called blue-green algae, are a group of prokaryotic microorganisms largely distributed in both terrestrial and aquatic environments. They produce a wide range of bioactive compounds that are mostly used in cosmetics, animal feed and human food, nutraceutical and pharmaceutical industries, and the production of biofuels. Nowadays, the research concerning the use of cyanobacteria in agriculture has pointed out their potential as biofertilizers and as a source of bioactive compounds, such as phycobiliproteins, for plant pathogen control and as inducers of plant systemic resistance. The use of alternative products in place of synthetic ones for plant disease control is also encouraged by European Directive 2009/128/EC.
- cyanobacteria
- plant pathogens
- fungi
- oomycetes
- antifungal activity
- biocontrol
- plant-induced resistance
- plant biostimulants
- cyanobacteria cultivation
1. Introduction
2. Plant Pathogen Control
2.1. Plant Pathogens
2.2. Cyanobacteria as Biocontrol Agents
2.2.1. In Vitro Studies and Mechanisms of Fungal and Oomycete Growth Inhibition
| Cyanobacterium | Extract/Culture Filtrate 1 | Plant Pathogen | Reference |
|---|---|---|---|
| Chroococcales | |||
| Microcystis aeruginosa | ME | Aspergillus carbonarius, A. niger | [43] |
| ETH | A. flavus, A. niger, A. parasiticus | ||
| AC | A. flavus, A. niger, Fusarium proliferatum | ||
| MC | A. flavus, A. parasiticus, F. proliferatum | ||
| DE | A. carbonarius, A. flavus, A. niger, A. ochraceus, A. westerdijkiae, F. proliferatum, F. verticillioides, Penicillium verrucosum | ||
| EA | A. carbonarius, A. flavus, A. niger, A. westerdijkiae, F. verticillioides | ||
| Nostocales | |||
| Anabaena spp. | CFILT | Alternaria solani, Drechslera oryzae, Fusarium moniliforme, F. solani, Macrophomina phaseolina, Pythium aphanidermatum | [17] |
| Anabaena sp. | PE | Alternaria alternata | [47] |
| Anabaena sp. | ME | Aspergillus flavus | [44] |
| A. cylindrica | ME | A. flavus | [44] |
| A. oscillarioides | CFILT, L | F. moniliforme, F. oxysporum f. sp. lycopersici, Pythium debaryanum, Rhizoctonia solani | [21] |
| A. solitaria | ME | Alternaria alternata | [47] |
| A. variabilis | CFILT, L | F. moniliforme, F. oxysporum f. sp. lycopersici, P. debaryanum, R. solani | [21] |
| A. variabilis | CFILT | F. oxysporum f. sp. lycopersici | [22] |
| A. laxa clones | CFILT | Pythium aphanidermatum | [51] |
| Calothrix brevissima | PE | Alternaria alternata | [47] |
| ME | A. alternata, Botrytis cinerea, F. oxysporum | [47] | |
| Fischerella sp. | ME | Aspergillus flavus | [44] |
| Nodularia sp. | ME | F. oxysporum | [47] |
| Nostoc sp. | ME | A. flavus | [44] |
| Nostoc strain ATCC 53789 | ME | Armillaria sp., Fusarium solani, F. oxysporum f. sp. melonis, Penicillium expansum, Phytophthora cambivora, P. cinnamomi, Rhizoctonia solani, Rosellinia sp., Sclerotinia sclerotiorum, Verticillium albo-atrum | [48] |
| Nostoc strain UTEX 2493 | ME | Rosellinia sp. | [48] |
| N. calcicula | ME | Aspergillus flavus | [44] |
| N. commune | ME | F. oxysporum f. sp. lycopersici | [50] |
| N. commune | PE | Phytophthora capsici, Pythium ultimum | [47] |
| ME | F. oxysporum, P. capsici | [47] | |
| N.commune | PE | Alternaria alternata | [47] |
| N.commune | ME | A. niger | [46] |
| PE | A. flavus, A. niger | [46] | |
| N.entophytum | AC, CHL, ME | R. solani | [52] |
| N. linckia | ME | F. oxysporum f. sp. lycopersici | [49] |
| N.muscorum | ME | A. alternata, B. cinerea, Colletotrichum gleosporioides | [47] |
| N.muscorum | CFILT | Aspergillus flavus, A. niger, Fusarium microsporium, Penicillium sp. | [45] |
| N.muscorum | AC, CHL, ME | R. solani | [52] |
| Scytonema sp., S. hofmanni |
ME | A. flavus | [44] |
| Oscillatoriales | |||
| Arthrospira platensis | PBPs | B. cinerea | [53] |
| Lyngbya lutea | W | A. niger | [46] |
| ME | A. niger, Colletotrichum musae, F. oxysporum | ||
| nPROP | A. flavus, F. oxysporum | ||
| PEE | A. niger, C. musae, F. oxysporum | ||
| Oscillatoria amphibia | W | A. flavus, C. musae | [46] |
| ME | F. oxysporum | ||
| nPROP | A. flavus, C. musae, F. oxysporum | ||
| O. angustissima | PE | C. gleosporioides, F. oxysporum | [47] |
| O. limosa | W | A. niger, C. musae | [46] |
| ME | A. flavus, A. niger, C. musae, F. oxysporum | ||
| nPROP | A. flavus, A. niger, C. musae, F. oxysporum | ||
| O. ornata | ME | A. flavus, A. niger, C. musae, F. oxysporum | |
| nPROP | C. musae, F. oxysporum | ||
| PEE | A. niger | ||
| O. tenuis | PE | A. alternata, P. capsici | [47] |
| ME | P. capsici | ||
| Phormidium autumnale | ME | F. oxysporum f. sp. lycopersici | [49] |
| P. tenue | W | A. niger, C. musae, F. oxysporum | [46] |
| ME | A. niger, F. oxysporum | ||
| nPROP | C. musae, F. oxysporum | ||
| PEE | A. niger, P. lilacimus | ||
| Trichodesmium hildebrantii | W | C. musae | [46] |
| ME | A. niger, C. musae, F. oxysporum | ||
| nPROP | A. flavus, A. niger, C. musae, P. lilacimus | ||
| PEE | C. musae, F. oxysporum | ||
| Synechococcales | |||
| Synechococcus elongates | W | C. musae | [46] |
| ME | A. niger, C. musae, F. oxysporum | ||
| nPROP | A. flavus, A. niger, P. lilacimus | ||
| PEE | C. musae, F. oxysporum | ||
| Synechocystis sp. | W | A. flavus, A. niger, C. musae, P. lilacimus | [46] |
| ME, nPROP | A. flavus, A. niger, C. musae, P. lilacimus, F. oxysporum | ||
| PEE | C. musae |
2.2.2. In Vivo Studies and Mechanisms of Biocontrol Ability
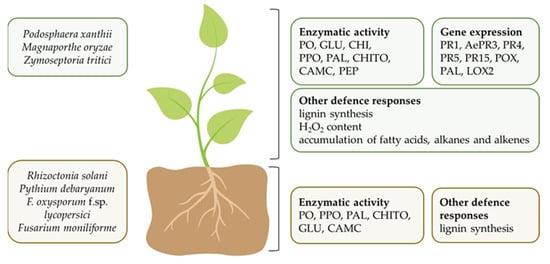
| Cyanobacterium | Extract/ Biomass/ Compound 1 |
Plant/ Treatment 3 |
Pathogen Control 4/ Plant Defense Responses 5 |
Reference |
|---|---|---|---|---|
| Nostocales | ||||
| Anabaena laxa | Biomass culture |
Coriander GS |
Shoot and root: PO activity; shoot: GLU activity | [57] |
| Cumin GS |
Shoot and root: PO activity | |||
| Fennel GS |
Shoot: PO activity | |||
| A. minutissima | W | Zucchini L |
Podosphaera xanthii (25%) CHI, GLU, PO activity, isoforms of CHI, GLU, PO |
[56] |
| W | Cucumber L |
P. xanthii (31%) PR1, AePR3 genes |
[24] | |
| W | Tomato S |
Rhizoctonia solani Seedling: CHI activity, lignin content |
[20] | |
| PBPs | Tomato F |
Botrytis cinerea cutin and pectin preservation |
[23] | |
| POL | Strawberry F |
B. cinerea | [55] | |
| A. variabilis | Biomass 2 | Tomato GS |
Pythium debaryanum, R. solani, Fusarium moniliforme, F. oxysporum f. sp. lycopersici | [21] |
| A. variabilis | Biomass 2 | Tomato seedling |
F. oxysporum f. sp. lycopersici (100%) GLU, PPO, PAL activity |
[22] |
| Calothrix elenkinii | Biomass culture |
Coriander GS |
Shoot and root: PO activity | [57] |
| Cumin GS |
Shoot and root: PO activity | |||
| Fennel GS |
Shoot: PO, GLU activity | |||
| C. elenkinii | Biomass | Rice GS |
Shoot and root: PPO, PAL, PO, CHITO, GLU, CAMC activity | [61] |
| C. elenkinii | Biomass | Rice GS |
Magnaporthe oryzae (50%) Leaf: PO, PPO, PAL, PEP activity |
[60] |
| Nostoc linkia | Biomass | Tomato GS |
F. oxyporum f. sp. lycopersici | [49] |
| N. punctiforme | Medium culture | Arabidopsis thaliana | WRKY | [58] |
| Nostoc-Anabaena consortium | Biomass | Rice GS |
Magnaporthe oryzae (69%) PPO activity |
[60] |
| Oscillatoriales | ||||
| Arthrospira platensis | DB | Wheat L |
Zymoseptoria tritici (~70%) PR4, PR5, PR15, PO, PAL, LOX genes |
[62] |
| A. platensis | PBPs | Tomato F |
B. cinerea | [53] |
| A. platensis | POL | Tomato L |
PAL, CHI, GLU, PO activity; H2O2 content; accumulation of fatty acids, azelaic acid, alkanes, alkenes, other metabolites | [63] |
3. Biostimulant Effects
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae8010058
References
- Schopf, J.W. The fossil record of cyanobacteria. In Ecology of Cyanobacteria II; Whitton, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 15–36.
- Blankenship, R.E. Origin and early evolution of photosynthesis. Photosynth. Res. 1992, 33, 91–111.
- Buick, R. The antiquity of oxygenic photosynthesis: Evidence from stromatolites in sulphate-deficient archaean lakes. Science 1992, 255, 74–77.
- Barsanti, L.; Gualtieri, P. General Overview. In Algae: Anatomy, Biochemistry, and Biotechnology; Barsanti, L., Gualtieri, P., Eds.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 1–6.
- Dvořák, P.; Casamatta, D.A.; Hašler, P.; Jahodářová, E.; Norwich, A.R.; Poulíčková, A. Diversity of the Cyanobacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 3–17.
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223.
- Stal, L.J. Cyanobacteria: Diversity and versatility, clues to life in extreme environments. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; Volume 11, pp. 661–682.
- Whitton, B.A.; Potts, M. Ecology of Cyanobacteria II: Their Diversity in Space and Time; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2012; pp. 1–13.
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86.
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97.
- Mantzouki, E.; Visser, P.M.; Bormans, M.; Ibelings, B.W. Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat. Ecol. 2016, 50, 333–350.
- Herrero, A.; Flores, E. The Cyanobacteria: Molecular Biology, Genomics and Evolution; Caister Academic Press: Sevilla, Spain, 2008; p. 484.
- Boden, J.S.; Konhauser, K.O.; Robbins, L.J.; Sánchez-Baracaldo, P. Timing the evolution of antioxidant enzymes in cyanobacteria. Nat. Commun. 2021, 12, 4742.
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs 2019, 17, 320.
- Shah, S.A.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.-W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Mar. Drugs 2017, 15, 354.
- Carpine, R.; Sieber, S. Antibacterial and antiviral metabolites from cyanobacteria: Their application and their impact on human health. Curr. Res. Biotechnol. 2021, 3, 65–81.
- Prasanna, R.; Nain, L.; Tripathi, R.; Gupta, V.; Chaudhary, V.; Middha, S.; Joshi, M.; Ancha, R.; Kaushik, B.D. Evaluation of fungicidal activity of extracellular filtrates of cyanobacteria—Possible role of hydrolytic enzymes. J. Basic Microbiol. 2008, 48, 186–194.
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant biostimulants from cyanobacteria: An emerging strategy to improve yields and sustainability in agriculture. Plants 2021, 10, 643.
- Berthon, J.-Y.; Michel, T.; Wauquier, A.; Joly, P.; Gerbore, J.; Filaire, E. Seaweed and microalgae as major actors of blue biotechnology to achieve plant stimulation and pest and pathogen—A review of the latest advances and future prospects. J. Agric. Sci. 2021, 1, 12.
- Righini, H.; Francioso, O.; Di Foggia, M.; Prodi, A.; Martel Quintana, A.; Roberti, R. Tomato seed biopriming with water extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens as a new agro-ecological option against Rhizoctonia solani. Sci. Hortic. 2021, 281, 109921.
- Chaudhary, V.; Prasanna, R.; Nain, L.; Dubey, S.C.; Gupta, V.; Singh, R.; Jaggi, S.; Bhatnagar, A.K. Bioefficacy of novel cyanobacteria-amended formulations in suppressing damping off disease in tomato seedlings. World J. Microbiol. Biotechnol. 2012, 28, 3301–3310.
- Prasanna, R.; Chaudhary, V.; Gupta, V.; Babu, S.; Kumar, A.; Singh, R.; Shivay, Y.S.; Nain, L. Cyanobacteria mediated plant growth promotion and bioprotection against Fusarium wilt in tomato. Eur. J. Plant Pathol. 2013, 136, 337–353.
- Righini, H.; Francioso, O.; Di Foggia, M.; Martel Quintana, A.; Roberti, R. Assessing the potential of the terrestrial cyanobacterium Anabaena minutissima for controlling Botrytis cinerea on tomato fruits. Horticulturae 2021, 7, 210.
- Righini, H.; Somma, A.; Cetrullo, S.; D’Adamo, S.; Flamigni, F.; Martel Quintana, A.; Roberti, R. Inhibitory activity of aqueous extracts from Anabaena minutissima, Ecklonia maxima and Jania adhaerens on the cucumber powdery mildew pathogen in vitro and in vivo. J. Appl. Phycol. 2020, 32, 3363–3375.
- Knogge, W. Fungal infection of plants. Plant Cell 1996, 8, 1711–1722.
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430.
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A review. Int. Microbiol. 2020, 23, 89–96.
- Morales, H.; Marín, S.; Rovira, A.; Ramos, A.J.; Sanchis, V. Patulin accumulation in apples by Penicillium expansum during postharvest stages. Lett. Appl. Microbiol. 2007, 44, 30–35.
- Bertero, A.; Moretti, A.; Spicer, L.J.; Caloni, F. Fusarium molds and mycotoxins: Potential species-specific effects. Toxins 2018, 10, 244.
- Carris, L.M.; Little, C.R.; Stiles, C.M. Introduction to Fungi. 2012. Available online: https://www.apsnet.org/edcenter/disandpath/fungalasco/intro/Pages/IntroFungi.aspx (accessed on 15 October 2021).
- Barberis, C.L.; Dalcero, A.M.; Magnoli, C.E. Evaluation of aflatoxin B1 and ochratoxin A in interacting mixed cultures of Aspergillus sections Flavi and Nigri on peanut grains. Mycotoxin Res. 2012, 28, 149–156.
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A global view on fungal infections in humans and animals: Opportunistic infections and microsporidioses. J. Appl. Microbiol. 2021, 131, 2095–2113.
- Jenkins, S.; Taylor, A.; Jackson, A.C.; Armitage, A.D.; Bates, H.J.; Mead, A.; Harrison, R.J.; Clarkson, J.P. Identification and expression of secreted in xylem pathogenicity genes in Fusarium oxysporum f. sp. pisi. Front. Microbiol. 2021, 12, 593140.
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324.
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: San Diego, CA, USA, 2005; p. 952.
- Batista, B.G.; de Chaves, M.A.; Reginatto, P.; Saraiva, O.J.; Fuentefria, A.M. Human fusariosis: An emerging infection that is difficult to treat. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200013.
- Lamour, K.H.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant. Pathol. 2012, 13, 329–337.
- Baker, K.F. Types of Rhizoctonia diseases and their occurrence. In Rhizoctonia solani: Biology and Pathology; Parmeter, J.R., Jr., Ed.; University of California Press: Berkeley, CA, USA, 1970; pp. 125–148.
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016; p. 486.
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; De Vicente, A.; Tores, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160.
- Keinath, A.P.; DuBose, V.B. Evaluation of fungicides for prevention and management of powdery mildew on watermelon. Crop Prot. 2004, 23, 35–42.
- Boddy, L. Pathogens of autotrophs. In The Fungi, 3rd ed.; Watkinson, S.C., Boddy, L., Money, N.P., Eds.; Academic Press: London, UK, 2016; pp. 245–292.
- Marrez, D.A.; Sultan, Y.Y. Antifungal activity of the cyanobacterium Microcystis aeruginosa against mycotoxigenic fungi. J. Appl. Pharm. Sci. 2016, 6, 191–198.
- Shishido, T.K.; Humisto, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.P.; Permi, P.; Andreote, A.P.D.; Fiore, M.F.; et al. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140.
- El-Sheekh, M.M.; Osman, M.E.H.; Dyab, M.A.; Amer, M.S. Production and characterization of antimicrobial active substance from the cyanobacterium Nostoc muscorum. Environ. Toxicol. Phar. 2006, 21, 42–50.
- Pawar, S.T.; Puranik, P.R. Screening of terrestrial and freshwater halotolerant cyanobacteria for antifungal activities. World J. Microbiol. Biotechnol. 2008, 24, 1019–1025.
- Kim, J.D. Screening of cyanobacteria (blue-green algae) from rice paddy soil for antifungal activity against plant pathogenic fungi. Microbiology 2006, 34, 138–142.
- Biondi, N.; Piccardi, R.; Margheri, M.C.; Rodolfi, L.; Smith, G.D.; Tredici, M.R. Evaluation of Nostoc strain ATCC 53789 as a potential source of natural pesticides. Appl. Environ. Microbiol. 2004, 70, 3313–3320.
- Alwathnani, H.A.; Perveen, K. Biological control of Fusarium wilt of tomato by antagonist fungi and cyanobacteria. Afr. J. Biotechnol. 2012, 11, 1100–1105.
- Kim, J.; Kim, J.-D. Inhibitory effect of algal extracts on mycelial growth of the tomato-wilt pathogen, Fusarium oxysporum f. sp. lycopersici. Mycobiology 2008, 36, 242–248.
- Gupta, V.; Prasanna, R.; Natarajan, C.; Srivastava, A.K.; Sharma, J. Identification, characterization, and regulation of a novel antifungal chitosanase gene (cho) in Anabaena spp. Appl. Environ. Microbiol. 2010, 76, 2769–2777.
- Osman, M.E.A.H.; El-Sheekh, M.M.; Metwally, M.A.; Ismail, A.E.W.A.; Ismail, M.M. Antagonistic activity of some fungi and cyanobacteria species against Rhizoctonia solani. Int. J. Plant Pathol. 2011, 2, 101–114.
- Righini, H.; Francioso, O.; Di Foggia, M.; Martel Quintana, A.; Roberti, R. Preliminary study on the activity of phycobiliproteins against Botrytis cinerea. Mar. Drugs 2020, 18, 600.
- Moreno, A.B.; Martínez del Pozo, Á.; Borja, M.; San Segundo, B. Activity of the antifungal protein from Aspergillus giganteus against Botrytis cinerea. Phytopathology 2003, 93, 1344–1353.
- Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299.
- Roberti, R.; Galletti, S.; Burzi, P.L.; Righini, H.; Cetrullo, S.; Perez, C. Induction of defence responses in zucchini (Cucurbita pepo) by Anabaena sp. water extract. Biol. Control 2015, 82, 61–68.
- Kumar, M.; Prasanna, R.; Bidyarani, N.; Babu, S.; Mishra, B.K.; Kumar, A.; Adak, A.; Jauhari, S.; Yadav, K.; Singh, R.; et al. Evaluating the plant growth promoting ability of thermotolerant bacteria and cyanobacteria and their interactions with seed spice crops. Sci. Hortic. 2013, 164, 94–101.
- Belton, S.; McCabe, P.F.; Ng, C.K.Y. The cyanobacterium, Nostoc punctiforme can protect against programmed cell death and induce defence genes in Arabidopsis thaliana. J. Plant Interact. 2021, 16, 64–74.
- Zheng, J.; Zhang, Z.; Tong, T.; Fang, Y.; Zhang, X.; Niu, C.; Li, J.; Wu, Y.; Xue, D.; Zhang, X. Genome-wide identification of wrky gene family and expression analysis under abiotic stress in barley. Agronomy 2021, 11, 521.
- Thapa, S.; Prasanna, R.; Ramakrishnan, B.; Mahawar, H.; Bharti, A.; Kumar, A.; Velmourougane, K.; Shivay, Y.S.; Kumar, A. Microbial inoculation elicited changes in phyllosphere microbial communities and host immunity suppress Magnaporthe oryzae in a susceptible rice cultivar. Physiol. Mol. Plant Pathol. 2021, 114, 101625.
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015, 171, 78–89.
- Le Mire, G.; Siah, A.; Marolleau, B.; Gaucher, M.; Maumené, C.; Brostaux, Y.; Massart, S.; Brisset, M.N.; Jijakli, M.H. Evaluation of λ-carrageenan, CpG-ODN, glycine betaine, Spirulina platensis, and ergosterol as elicitors for control of Zymoseptoria tritici in wheat. Phytopathology 2019, 109, 409–417.
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; El Arroussi, H. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930.
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754.
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782.
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587.
- Li, J.; Li, C.; Smith, S.M. Hormone Metabolism and Signaling in Plants; Academic Press Elsevier: London, UK, 2017; p. 597.
- Karthikeyan, N.; Prasanna, R.; Sood, A.; Jaiswal, P.; Nayak, S.; Kaushik, B.D. Physiological characterization and electron microscopic investigation of cyanobacteria associated with wheat rhizosphere. Folia Microbiol. 2009, 54, 43–51.
- Maqubela, M.P.; Mnkeni, P.N.S.; Malam Issa, O.; Pardo, M.T.; D’Acqui, L.P. Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility, and maize growth. Plant Soil 2009, 315, 79–92.
- Řezanka, T.; Palyzová, A.; Sigler, K. Isolation and identification of siderophores produced by cyanobacteria. Folia Microbiol. 2018, 63, 569–579.
- Shukia, S.P.; Singh, J.S.; Kashyap, S.; Giri, D.D.; Kashyap, A.K. Antarctic cyanobacteria as a source of phycocyanin: An assessment. Indian J. Mar. Sci. 2008, 37, 446–449.
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80.
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/biostimulant-market-1081.html (accessed on 9 October 2021).
- Arnau, L. Techno-Economic Feasibility Study for the Production of Microalgae Based Plant Biostimulant. Master’s Thesis, KTH, Royal Institute of Technology School of Chemical Science and Engineering, Stockholm, Sweden, 2016.
- El Boukhari, M.E.M.E.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359.
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, S.P.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Yunus Khan, T.M.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782.
- Romero Villegas, G.I.; Fiamengo, M.; Acién Fernández, F.G.; Molina Grima, E. Outdoor production of microalgae biomass at pilot-scale in seawater using centrate as the nutrient source. Algal Res. 2017, 25, 538–548.
- Aghofack-Nguemezi, J.; Schinzoumka, P.A.; Tatchago, V. Effects of extracts or powder of Jatropha curcas and Spirulina platensis on the growth and development of tomato plant. J. Appl. Biosci. 2015, 90, 8413–8420.
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273.
- Mógor, Á.F.; Ördög, V.; Pereira Lima, G.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460.
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453.
- Toribio, A.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Moreno, J. Prospection of cyanobacteria producing bioactive substances and their application as potential phytostimulating agents. Biotechnol. Rep. 2020, 26, e00449.
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244.
- Žižková, E.; Kubeš, M.; Dobrev, P.I.; Přibyl, P.; Šimura, J.; Zahajská, L.; Drábková, L.Z.; Novák, O.; Motyka, V. Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann. Bot. 2017, 119, 151–166.
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346.
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2017, 30, 1061–1071.
