Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microplastics (MPs) can also function as indicators for metals, antibiotics, toxic chemicals, pathogenic bacteria Harmful Algal Bloom (HAB)-forming dinoflagellates across the continents, especially through ballast water, serving as “hotspots” in ballast waters for developing and spreading multiple drug-resistant human pathogens through co-selection mechanisms.
- microplastics
- water sources
- environmental pollution
- marine environment
- freshwater
- toxicological effects
1. Compounds Detected in Microplastic Samples
1.1. Sizing and Characterization of Microplastics
MPs have been mainly detected at beaches and surface layers of lakes in significant quantities [1][2][3][4]. Statistically significant particle size reduction has been reported from riverine to coastal areas, where the prevailing polymer types are that of polyethylene (PE), polypropylene (PP) [5], as well as polyester and synthetic dye [6].
MPs abundance can be traced to closely populated areas, as well as at municipal and industrial effluent discharges [7]. In this context, MPs in sediments were reported and their MP concentrations were determined, for the first time, in the surface sediment of seven streams around the lagoon of Bizerte (Northern Tunisia), using a saturated NaCl flotation technique. Categorization of MPs was based on type, color, and size using a stereoscopic microscope [7]. Precisely, sampling certainly contains secondary MPs in the descending quantities of fibers, fragments, and films, while the reported colors are black, clear, white, red, blue, green, and yellow for fibers; white, blue, black, and red for fragments; and red, white, clear, green, and blue-black for films. In the same study, it was reported that MP particles in sampling ranged from 0.2 to 5.0 mm in length. Different stream-sites of sampling revealed significant MPs type differences, ranging at Khima stream (min concentration of 2340 ± 227.15 items kg −1 dry weight) up to Jedara stream (max concentration of 6920 ± 395.98 items kg−1 dry weight) [7].
While in the relevant literature it is not exactly reported which synthetic substances can be classified as MPs, the sources of MPs detection in water resources in the environment can be precisely determined as plastic granules, or pellets, serving as a raw material for manufacturing plastic sheets or ready-to-use items, while the cosmetic industry also uses microgranules (microspheres, nanospheres, microcapsules, nanocapsules) [8]. An indicative classification according to the dimensional range of MPs particle sizing, in alignment with relevant methods that measure their distribution in water resources, is represented in Figure 1.
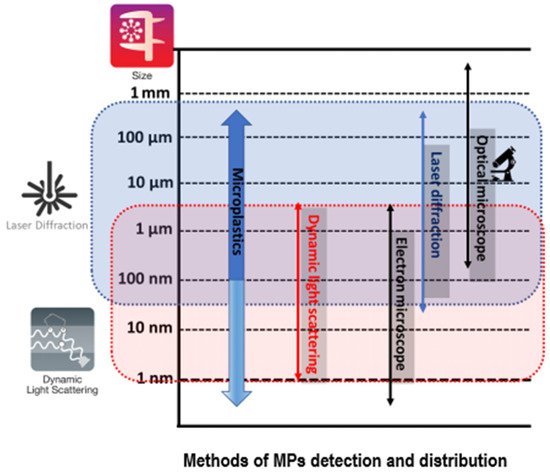
Figure 1. Methods of MPs detection and distribution, in alignment with their particle size. Source: Lee and Chae [9] (p. 6).
Based on Figure 1, there is no scientific affirmation about the exact particle size defining the MP particles, whereas there is a widely accepted agreement that MPs are those particles having a range size 0.5–5 mm and attributing them in their larger dimension. Another proposition of MP sizing is proposing a lower limit close to 0.3 mm, taking into consideration the widespread techniques of water sampling using zooplankton nets of 333 μm mesh size [8]. However, it remains unclear in defining the maximum particle size of MPs, being a continuing matter of discussion [8]. It is scientifically correct to propose that particles ranged at 0.5–5 mm should be conventionally considered as a typical range of definition, since substantial technical difficulties have to be confronted while analyzing particles smaller than 0.5 mm [8].
Based on Figure 1 and Table 2, it can be stated that among the MP detection methods, vibrational spectroscopy and thermosanalysis both necessitate diversified preparation of sampling with reference to specified methodology. A typical application of such a sampling can include the protocol of sampling, the filtration, the digestion of acid, and the density separation. This procedural analysis is case-specific in alignment with the aquatic environment: marine, wastewater effluent, surface water, bottled water [9]. Moreover, the fact that interfering materials are different in each matrix cannot be undermined, thus, a variety of different samples should follow preparation protocols for each case. The scope of such a formalized analysis is the running of accurate and reliable information, based on the size distribution, the chemical composition, and the concentration by mass and particle numbers. Subsequently, a multiple analytical technique should be executed, compared to the traditional analytical approach of a single methodology. MP analysis showed that polymer identification, the particle size, and the MP abundance can be satisfactorily investigated by FTIR and Raman analyses, while mass concentration can be measured by GC-MS coupled with thermal decomposition [9].
Table 1. Representative types of MPs and the corresponding characteristic compounds, limit of detection (LOD), retention index (LRI), mass to charge ration (m/z), and density.
| Polymeric Substance |
Characteristic Compound 1 | LRI 2 | m/z 3 | LOD (μg) | Density (g/cm3) |
|---|---|---|---|---|---|
| PP | 2,4-dimethyl-I-heptene | 846 | 70 | 0.027 | 0.85–0.92 |
| PE (LD and HD) | I-Decene (C10) | 993 | 83;97 | 0.070 | 0.89–0.97 |
| PA-6 | ε-caprolactam | 980 | 113 | 0.110 | 1.14 (PA–66) |
| PS | Styrene | 898 | 78;104 | 0.003 | 1.04–1.08 |
| PMMA | Methyl methacrylate | 743 | 41;69;100 | 0.029 | 1.18–1.20 |
| uPVC or PVC | Napthalene | 1206 | 128 | 0.592 | 1.16–1.41 |
| PET | Acetophenone | 1076 | 51;77;105 | 0.015 | 1.38–1.70 |
1 Marker compound used to calculate limit of detection (LOD); 2 Retention index; 3 Mass to charge ratio (ion indicator) Source: [20,59].
Another criterion of characterizing MPs’ intake and environmental damage caused to fisheries is the maximum number of MPs ingested by each fish, which is strongly correlated to exposure, being measured on the distance counted from the source of the river [6]. The integrated representation of the process followed for MPs’ identification and quantification is visualized at Figure 2.
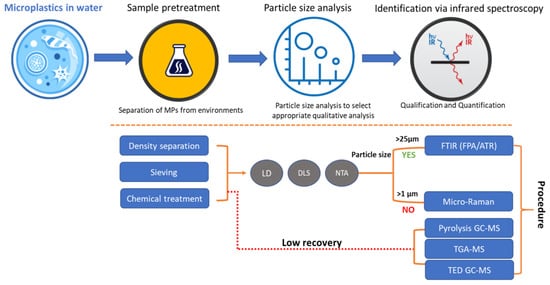
Figure 2 Graphical representation of quantitative/qualitative analyses of MPs identification. Source: Lee and Chae [9] (p. 12).
Based on Figure 2, it has been shown that the naturally abundant polymers of polypropylene and polyethylene can be mainly detected by FTIR analysis [7]. The quantification of MPs by FTIR analysis showed that the predominant MP (in abundance terms) is the polyamides (up to 53.3%), followed by polyethylene and polypropylene (up to 17.1%). Comparably, the planting compounds showed the abundance of natural fragments such as cellulose and wood at proportions of more than 45.8% and 18.8%, respectively [10]. In a similar study, the plastic fragments were identified as being of the most prevalent types of MPs, accounting for 68.5% among all MPs found [11]. Consistent with other studies, as shown in Table 2, it can be argued that MPs’ abundance can be positively correlated with fragments’ abundance at an increased catchment urbanization, being also commonly composed by the polymeric substances of PET, PP, PE, PS, PAM, and PVC at various mass proportions [9][12].
| MP Characteristics | PP | PE | PS, PAM, PVC |
|---|---|---|---|
| Atlantic Ocean water, 1.15 particles/m3 |
Polyester: 49% | PA/acrylic polyester: 43% | Analysis: FTIR |
| Atlantic Ocean water, size distribution | 0.25–0.5 mm: highest number | 0.25–0.5 mm: highest number | - |
| Density (g/cm3) | 0.85–0.92 | 0.89 (LDPE); 0.94 (HDPE) | 1.04–1.08 (PS) 1.16–1.41 (PVC) |
| European coastal waters, 13–501 particles/m3 |
48% | 48% | 4% PS; 11% PA Analysis for concentration and particle size distribution: slight microscopy |
| Size distribution | 5–10 μm: 30–40% | >10 μm:<10% | >100 μm: <2% |
| Size distribution | 1–5 μm: 25–60% | 5–10 μm: 30–50%; 10–50 μm: 10–60%; 50–100 μm: 0% | |
| Size distribution (μm) | - | - | 62.38 (PS); 53.58 (PA); 59.97 (PVC) |
| Surface area (m2/g) | - | - | 4.13 (PS); 9.51 (PA); 5.29 (PVC) |
| Surface seawater, 545 particles/m3 |
11.1% | 77.8% | PE/EA: 11.1% Analysis: FTIR spectroscopy |
| Surface seawater, Size distribution |
<0.5 mm: highest number |
<0.5 mm:highest number |
0.5–5 mm |
| Total pore volume (cm3/g) | - | - | 0.044 (PS); 0.090 (PA); 0.051 (PVC) |
| WTP drinking water | 16–33% | 0–35% | PAM, PVC < 10% |
| WTP raw water | 16–26% | 0–24% | PS, PAM, PVC < 10% Analysis: 10 µm: FTIR; <10 µm: Raman; SEM |
Notation: Polyamide (PA); polypropylene (PP); low-density polyethylene (LDPE); high-density polyethylene (HDPE); polystyrene (PS); nylon 66 (PA 66); poly(methyl methacrylate) (PMMA); polyvinyl chloride (PVC); polyethylene terephthalate (PET); polyacrylamide (PAM); poly Ether Amide (PE/EA); water treatment plant (WTP).
1.2. Microbiology and Toxicological Conditions of Microplastics
From a microbiology point of view, MP particles of ~40 μm can be related to a low concentration level of 1% of the food particles, being reformulated to an average of ~30 particles in the digestive tract. This correlation resembles a high MP contamination but it is still reflecting on a natural situation. Interestingly, neither increased mortality nor morphological changes in the body and tail spine lengths and width or reproductive abnormalities have been reported for adult water fleas of Daphnia magna (a freshwater zooplankton species) [13]. It is also noteworthy that the channeling of MP particles in marine environments is unavoidably influenced by the characteristics of size, shape, type, and age, while those rather weak effects detected in a laboratory may result in reduced fitness in a natural multi-stressor environment [13].
From a toxicological point of view, it can be stated that MPs are prone to absorbing organic contaminants, metals, and pathogens from marine environments into organisms, thus, exacerbating their toxicology features while interacting and causing greater toxic effects. While it has been well known that in marine environments, MPs’ accumulation commonly occurs, MPs are ingested from marine vertebrates that seabirds use as bioindicators [14]. Therefore, MP debris causes asphyxiation through drowning, but a restriction in feeding intensifies the symptoms of starvation, skin abrasions, and skeletal injuries. Furthermore, MP ingestion can block the guts, causing injuries of the gut lining, morbidity, and mortality. Subsequently, small-sized MPs can accelerate their translocation across gastro-intestinal membranes of organisms via endocytosis-like mechanisms into tissues’ and organs’ distribution. Furthermore, MPs can increase dysregulation of gene expression required in biological systems, while the malfunctions caused are those of MPs’ induction of oxidative stress, immunological responses, genomic instability, disruption of endocrine system, neurotoxicity, reproductive abnormities, embryotoxicity, and trans-generational toxicity [14].
Conclusively, research focus should be based on the recognition of new sources of MPs that possibly represent major environmental inputs, compared to those previously considered. In addition to the already renowned sources, pollutants such as the MPs can be also released by embrittlement of plastic litter, microbeads released from personal care products, washing of synthetic clothes, abrasion of vehicle tires, or weathering of different types of paints [15].
2. Discussion, Environmental Considerations, and Challenges
The literature coverage of this review study certainly evidenced the fact of MPs’ release into water resources, mainly reported in stormwaters and human-related activities, including sport and recreational activities. In recent years, global research has focused on MPs in marine ecosystems, but data on presence, monitoring, and assessment in freshwater environments are still scarce [16]. This research limitation can closely approach the occurrence, distribution, and chemical composition of MP pollution, mainly in European ponds, aquifers, and water resources [16]. In this research context, a systematic flow-chart of the presence of MPs in surface waters through land and sea origins is presented in Figure 3.
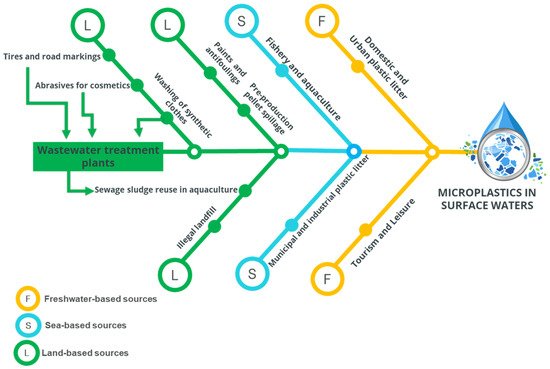
Figure 3. Flow-chart of the presence of microplastics in surface waters trough land and sea origins. Source: Modified and enhanced from Galafassi et al. [15] (p. 133499).
Figure 3 collectively represents that critical and challenging issues for researchers are the conceptualization of MP as a complex, dynamic mixture of land-, sea-, and freshwater-based sources. In the relevant literature, it was shown that polymers and additives, to which organic material and contaminants can successively bind to form an ecocorona, can increase the density and surface charge of particles and change their bioavailability and toxicity. However, there exists a major research gap on ecocorona formulation and in-field applicability in natural water resources [17]. It is also noteworthy that chronic exposure to MPs is rarely lethal, but it adversely affects individual animals, since MPs reduce food and deplete energy stores, with knock-on effects for fecundity and growth. Therefore, it is important for researchers to discover and comprehend the exact ecological processes that affect altered behaviors, bioturbation, and behavioral changes of carbon flux to the deep ocean [17].
There is growing evidence that MP contamination is extended even to freshwater ecosystems, a fact that can lead future studies and risk assessments [7]. Moreover, reporting MPs in freshwater and other water sources suggests that the mechanism of secondary MPs can be delivered by diffuse sources of pollution, especially among rivers and other water bodies of circulating currents, no stagnant waters. Secondary MPs sources in these types of flowing systems are determining the food chain through their intake by fauna, thus, consecutive desorption, fate, and risks around world deserve further research attention both in field and laboratory studies [5][10]. These studies should explore the ways in which MPs are ingested by freshwater fish and, then, evidencing those factors that influence ingestion and exposure hazardous matter to fish and marine biota [6].
From a methodological point of view, MPs’ identification, comparison of procedures, limitations, and applicability of supplementary types of analysis such as FTIR, Raman spectroscopy, and thermo-analytical methods are recommended [9]. In this respect, future research on vibrational spectroscopy for MP detection is anticipated to minimize misidentification of the potential MPs in the environment. The accuracy and the efficiency of natural sampling are factors of utmost importance of the quantitative analytical procedure, rather than the (decreasing) analysis time efficiency. Moreover, it is analytically challenging to solidify the accuracy of identification of potential MPs traces by FTIR and Raman by the type and size of MPs being priority over decreasing analysis time, while considering a 25 mm filter area taking over 7 h to analyze [9]. There is a wider possible application of FPA-micro-FTIR and it has a feasible application to water and wastewater treatment. Analytical improvements contain the tracing capability, the accuracy, and the time efficiency of FTIR, while electron-multiplying charged coupled device detector (EM CCD) showed a low signal to noise ratio [9].
Advancements of analytical techniques can be further recommended for degrading (by weathering) MPs to be collected at spectral libraries, enabling the identification and the quantification of such degraded MPs in samples analyzed. Moreover, IR and Raman analysis can be designed and developed for the better preparation of samples, since minimizing the chemical modification of the MPs samples can cause a misinterpretation by the IR spectra [9]. Another analytical challenge of complementary to μ-Raman spectroscopy is that Py-GC/MS pigment, containing particles, fibers, and sea sediment particles, are all identified as plastics [18]. From a technical point of view, the foundation of systematic protocols for MPs analysis is also suggested [9], focusing on: (a) developing water treatment strategies, (b) setting effective limits for MPs, (c) making thorough conventions of what types of water management are linked to what types of MPs, which are both (water- and MPs- types) characterized as hazardous materials [19].
Another research orientation should provide a better understanding of the MPs-carriers sorption mechanism as well as the desorption behavior under different environmental conditions in water resources [20]. It is noteworthy that water sensing devices can support clean and safe water bodies [21], while the adsorption performance of heavy metals by polymeric MPs can better simulate the surface attachment models developed at different kinds of pollutants [20].
Polymeric MPs can be proven as indicators of emerging environmental pollution, acting as carriers for bacterial colonization and propagation of particularly harmful microorganisms, leading to ecological risks attributed due to high stability, pathogenicity, and stress tolerance of the bacterial communities on MPs [22]. Therefore, new insights can pave the way for understanding bacterial dynamics on polymeric MPs in urban water environments [23]. However, future research should confront MPs pollution with susceptibility regarding MPs’ pollution in urban wetlands, stressing out the ubiquitous nature of the prevailing MP fragments, indicating that plastic litter degradation plays a significant role of MPs’ sourcing in urban environments and industrial areas [11].
It is also imperative that experimental results should be confirmed by model-based studies, investigating the extent to which MP concentrations are negligible, or not, for the overall pollutant uptake of freshwater indicators, such as zooplankton species, with water as an additional uptake pathway [24]. Such research paths can reveal the contradictions between the level and the severity of MPs presented in water sources, thus, encouraging future studies to:
- (a)
-
further and fully investigate how MPs are considered as dominant anthropogenic pollutants of ecological risk,
- (b)
-
support the primary scope of science and society in tackling such a global environmental issue in the future [17],
- (c)
- (d)
-
reinvent plastics production under the environmental considerations and the social provisions for radical modes of eco-design plastics production, biodegradable plastics production, as well as a circular thinking of manufacturing production, making the used plastic products able to undergo a second round of use after recovering and recycling. Moreover, legislative framework updating and WWTPs’ adaptation to the aforementioned directions should be shown to be vital tools to endorse those safety regulations of MP pollution decrease in the contexts of circular economy and the employment of effective practices to control the plastic waste crisis [32][33].
This entry is adapted from the peer-reviewed paper 10.3390/su14020828
References
- Faure, F.; Corbaz, M.; Baecher, H.; De Alencastro, L.F. Pollution due to plastics and microplastics in lake Geneva and in the Mediterranean sea. Arch. Des Sci. 2012, 65, 157–164.
- Karthik, R.; Robin, R.S.; Purvaja, R.; Ganguly, D.; Anandavelu, I.; Raghuraman, R.; Hariharan, G.; Ramakrishna, A.; Ramesh, R. Microplastics along the beaches of southeast coast of India. Sci. Total Environ. 2018, 645, 1388–1399.
- Yabanlı, M.; Yozukmaz, A.; Şener, İ.; Ölmez, Ö.T. Microplastic pollution at the intersection of the Aegean and Mediterranean Seas: A study of the Datça Peninsula (Turkey). Mar. Pollut. Bull. 2019, 145, 47–55.
- Bridson, J.H.; Patel, M.; Lewis, A.; Gaw, S.; Parker, K. Microplastic contamination in Auckland (New Zealand) beach sediments. Mar. Pollut. Bull. 2020, 151, 110867.
- Abeynayaka, A.; Kojima, F.; Miwa, Y.; Ito, N.; Nihei, Y.; Fukunaga, Y.; Yashima, Y.; Itsubo, N. Rapid sampling of suspended and floating microplastics in challenging riverine and coastal water environments in Japan. Water 2020, 12, 1903.
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194.
- Toumi, H.; Abidli, S.; Bejaoui, M. Microplastics in freshwater environment: The first evaluation in sediments from seven water streams surrounding the lagoon of Bizerte (Northern Tunisia). Environ. Sci. Pollut. Res. 2019, 26, 14673–14682.
- Zobkov, M.B.; Esiukova, E.E. Microplastics in a Marine Environment: Review of Methods for Sampling, Processing, and Analyzing Microplastics in Water, Bottom Sediments, and Coastal Deposits. Oceanology 2018, 58, 137–143.
- Lee, J.; Chae, K.J. A systematic protocol of microplastics analysis from their identification to quantification in water environment: A comprehensive review. J. Hazard. Mater. 2021, 403, 124049.
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504.
- Townsend, K.R.; Lu, H.C.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561.
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209.
- Imhof, H.K.; Rusek, J.; Thiel, M.; Wolinska, J.; Laforsch, C. Do microplastic particles affect Daphnia magna at the morphological, life history and molecular level? PLoS ONE 2017, 12, 0187590.
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74.
- Galafassi, S.; Nizzetto, L.; Volta, P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019, 693, 133499.
- Scopetani, C.; Chelazzi, D.; Cincinelli, A.; Esterhuizen-Londt, M. Assessment of microplastic pollution: Occurrence and characterisation in Vesijärvi lake and Pikku Vesijärvi pond, Finland. Environ. Monit. Assess. 2019, 191, 1–17.
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 116.
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676.
- Naik, R.K.; Naik, M.M.; D’Costa, P.M.; Shaikh, F. Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: A potential risk to the marine environment and human health. Mar. Pollut. Bull. 2019, 149, 110525.
- Zhang, W.; Zhang, L.; Hua, T.; Li, Y.; Zhou, X.; Wang, W.; You, Z.; Wang, H.; Li, M. The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment. Environ. Pollut. 2020, 257, 113440.
- Dimaano, R.J.D.; Albo, A.C.; Adion, A.X.Z.M.; Brucal, J.K.H. Antipara (Analysis of tiny particles in aquatic environment): A water scanning device for microplastics. Int. J. Adv. Trends Comput. Sci. Eng. 2020, 9, 5217–5221.
- Wu, N.; Zhang, Y.; Zhao, Z.; He, J.; Li, W.; Li, J.; Xu, W.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary. Sci. Total Environ. 2020, 708, 134876.
- Wang, L.; Luo, Z.; Zhen, Z.; Yan, Y.; Yan, C.; Ma, X.; Sun, L.; Wang, M.; Zhou, X.; Hu, A. Bacterial community colonization on tire microplastics in typical urban water environments and associated impacting factors. Environ. Pollut. 2020, 265, 114922.
- Rehse, S.; Kloas, W.; Zarfl, C. Microplastics reduce short-term effects of environmental contaminants. Part I: Effects of bisphenol a on freshwater zooplankton are lower in presence of polyamide particles. Int. J. Environ. Res. Public Health 2018, 15, 280.
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68.
- Zhang, Y.; Liang, J.; Zeng, G.; Tang, W.; Lu, Y.; Luo, Y.; Xing, W.; Tang, N.; Ye, S.; Li, X.; et al. How climate change and eutrophication interact with microplastic pollution and sediment resuspension in shallow lakes: A review. Sci. Total Environ. 2020, 705, 135979.
- Zamparas, M.; Kyriakopoulos, G.L.; Drosos, M.; Kapsalis, V.C.; Kalavrouziotis, I.K. Novel Composite Materials for Lake Restoration: A New Approach Impacting on Ecology and Circular Economy. Sustainability 2020, 12, 3397.
- Zamparas, M.; Kyriakopoulos, G.L.; Kapsalis, V.C.; Drosos, M.; Kalavrouziotis, I.K. Application of novel composite materials as sediment capping agents: Column experiments and modelling. Desalin. Water Treat. 2019, 170, 111–118.
- Zamparas, M.; Drosos, M.; Deligiannakis, Y.; Zacharias, I. Eutrophication control using a novel bentonite humic-acid composite material BephosTM. J. Environ. Chem. Eng. 2015, 3, 3030–3036.
- Greaver, T.L.; Clark, C.M.; Compton, J.E.; Vallano, D.; Talhelm, A.F.; Weaver, C.P.; Band, L.E.; Baron, J.S.; Davidson, E.A.; Tague, C.L.; et al. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 2016, 6, 836–843.
- Gianni, A.; Zamparas, M.; Papadas, I.T.; Kehayias, G.; Deligiannakis, Y.; Zacharias, I. Monitoring and Modeling of Metal Concentration Distributions in Anoxic Basins: Aitoliko Lagoon, Greece. Aquat. Geochem. 2013, 19, 77–95.
- Kyriakopoulos, G.L.; Kapsalis, V.C.; Aravossis, K.G.; Zamparas, M.; Mitsikas, A. Evaluating Circular Economy under a Multi-Parametric Approach: A Technological Review. Sustainability 2019, 11, 6139.
- Calero, M.; Godoy, V.; Quesada, L.; Martín-Lara, M.Á. Green strategies for microplastics reduction. Curr. Opin. Green Sustain. Chem. 2021, 28, 100442.
This entry is offline, you can click here to edit this entry!
