Hydrogenases catalyze the reversible oxidation of H2, and are found in many organisms, including plants. One of the cellular effects of H2 is the selective removal of reactive oxygen species (ROS) and reactive nitrogen species (RNS), specifically hydroxyl radicals and peroxynitrite. Therefore, the function of hydrogenases and the action of H2 needs to be reviewed in the context of the signalling roles of a range of redox active compounds. Enzymes can be controlled by the covalent modification of thiol groups, and although motifs targeted by nitric oxide (NO) can be predicted in hydrogenases sequences it is likely that the metal prosthetic groups are the target of inhibition.
- HYDROGENASES
- PLANTS
- REDOX
- CHEMISTRY
- MOLECULAR HYDROGEN
1. Introduction
Treatment with H2 has been described as being beneficial for a range of human diseases [3,4], whilst in agriculture, application of H2 has been demonstrated to increase both crop health and yield [5], important factors that may prove beneficial for the arable and cattle-feed industries in particular. To illustrate, evidence shows that H2 is able to mediate root development and stress responses in plants, in response to heavy metals [6] and drought [7] in particular. It can also be used for improving post-harvest storage of crops, for example with kiwifruits [8]. Therefore, how organisms such as plants can be exposed to H2, and how they respond to it, is important to understand and may lead to better treatments and better yields in the future.
In light of ongoing research it is becoming clear that H2 should be seen as part of a suite of small reactive molecules that can influence and control cellular function. It has long been known that reactive oxygen species (ROS), such as superoxide anion (O2·−), hydrogen peroxide (H2O2) and hydroxyl radicals (·OH), are produced in cells and can impact activities in the intracellular and extracellular environs, during stress responses as an example [9]. Of further significance are the reactive nitrogen species (RNS), such as nitric oxide (·NO) and peroxynitrite (ONOO−) [10]. Hydrogen sulfide (H2S) too, is an important signalling molecule [11], often produced, along with ROS and RNS, during stress responses [12].
As these molecules can be generated by plant cells concomitantly, both temporally and spatially, it is likely that there would be an interplay between them [13], and also, interactions with H2. This crosstalk could, of course, be bidirectional wherein H2 may interfere with NO signalling, for example. Alternatively ROS and NO may also modify H2 metabolism. In either case, signalling events would be affected, and this would influence both short-term and long-term cellular activities.
H2 is an extremely small (MW 2.016 g/mol) and relatively inert molecule. As a consequence, it is hard to envisage how it can be perceived by a classical receptor protein, or how it could partake in the control of proteins through covalent modifications, as has been found for NO effects, typically through S-nitrosation [14]. However, H2 has been shown to have effects through the selective removal of reactive oxygen species (ROS), in particular hydroxyl radicals [15], and the scavenging of RNS, in particular peroxynitrite. H2 is also known to have effects through action on haem oxygenase enzymes (e.g., HO-1) [16]. It has also been mooted that the physical properties of H2 may mediate some of the effects seen in higher plants and animals [17]. However, we are currently far from a full understanding of how H2 interacts with cellular components and influences cellular activity, and a great deal of research into the molecular mechanisms of the interplay between molecular hydrogen and cellular systems will be required if we are to elucidate such complexities.
Cells may be exposed to H2 from both endogenous and exogenous sources. Exogenous sources, such as the arrival of H2 from the environment, are important to consider, especially as this may be the way H2 is used as a medication or agricultural treatment. However, many organisms are known to contain discrete hydrogenase enzymes responsible for the reversible oxidation of molecular hydrogen. Such enzymes are typically classified on the basis of their metal chelation properties e.g., Fe, FeFe, and NiFe [18], which may either generate or remove molecular hydrogen in cellular systems. In animals, the gut microflora is also an important source of H2 [19]; however, non-gut bacteria may also contribute to accumulation of H2 in biological systems [20].
2. Hydrogenases of Higher Plants
It has been known for a long time that hydrogen is metabolized by higher plants and that (FeFe)-hydrogenases exist in eukaryotic species, including plants [45]. These metallo-protein complexes appear to be able to catalyze both forward and backwards reactions, effectively removing or generating H2. They also seem to be involved in the appropriate biosynthesis of (Fe-S) clusters and the sensitivity to O2. Knockouts of encoding genes leads to poor plant development, with these enzymes appearing to be involved in the control of the cell cycle and sugar metabolism [46], as well as transcription control and in stress responses [47]. Eukaryotic hydrogenases are often referred to as NAR (nuclear architecture related) or GOLLUM (different oxygen levels influences morphogenesis) proteins. For example, in plants there is GOLLUM1 in Medicago truncatula [48] and AtNAR1 in Arabidopsis [49], although currently such naming appears to be interchangeable.
It is tempting, as with the hydrogenases in C. reinhardtii, to suggest that redox signalling molecules may have an impact on the action of higher plant hydrogenases. As with the HYDA1 and HYDA2 sequences above, if such enzymes are controlled by the presence of ROS, NO, or H2S, then thiol side groups may be the targets for modification. This may, for example, be for covalent modification with ROS or NO, but neither polypeptide sequence contains any –SNO motifs ((IL)-X-C-X-X-(DE) [37]) suggesting that control by NO by this means is unlikely. A brief analysis of the Arabidopsis AtNar1 sequence (Accession number: NM_117739) shows 13 cysteine residues. When aligned with the ferredoxin hydrogenase from Artemisia annua (sweet wormwood or qinghao: Accession number: PWA71961) there is 69% identity in the sequence. Interestingly, all but one of the cysteine residues is conserved, thereby evolution would suggest that they are retained for a reason.
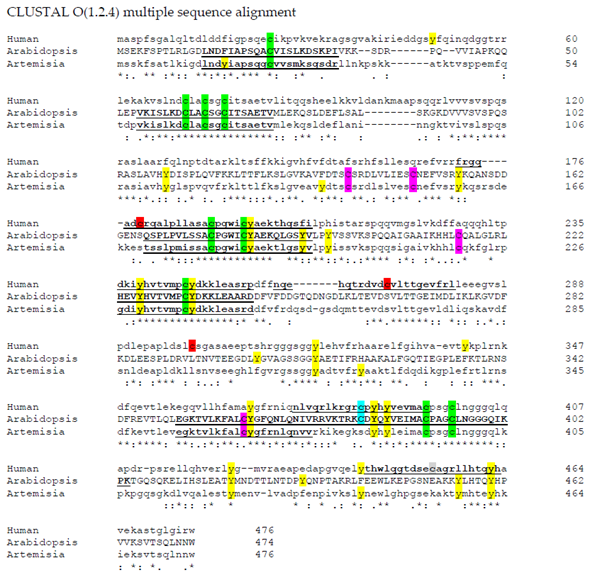
Aligning these plant sequences with the sequence for the human cytosolic iron-sulfur assembly component 3 isoform 1 (Accession number: NP_071938.1), using Clustal Omega [50], there are still nine conserved cysteine residues. Surprisingly, Cys 380 is conserved in Arabidopsis AtNAR1 but not in the Artemisia sequence, yet it is contained in the human sequence (Figure 1). The human sequence also contains three cysteine residues not found in the plant sequences, but still they do not fall in a –SNO conserved region. However, putting the sequence through the iSNO-PseAAC prediction tool [35] gives the data shown in Table 1. Four conserved cysteine residues in the three sequences investigated were predicted to be modified by NO. Using the Arabidopsis sequence numbers, these were Cys24, Cys177, Cys233, and Cys362. Interestingly, two of these are conversed also in the human sequence: Cys177 and Cys233 (Table 1 and Figure 1). Such conservation across a wide range of species suggests that they have a possible significant role and it may be that they are there for control through NO signalling. This is, of course, with the caveat that it has been suggested that the three-dimensional orientation of the amino acids is more important than the sequence in the region [36]. Furthermore, as discussed above, it is likely that the metal prosthetic groups are the target for NO, rather than the amino acid thiol side chains [38].
Figure 1. Amino acid sequence alignments of hydrogenases. Clustal Omega [50] was used to align three hydrogenase sequences: human cytosolic iron-sulfur assembly component 3 isoform 1(Accession number: NP_071938.1); AtNar1 (Accession number: NP_567496.4); ferredoxin hydrogenase from Artemisia annua (Accession number: PWA71961). C: Common to all sequences; C: common to just plant species; C: Common to human and Arabidopsis; C: unique to human; and Y: tyrosine residues. Sequences predicted to become –SNO using the iSNO-PseAAC prediction tool [35] are underlined and in bold. –SNO: nitrosated thiol.
Table 1. S-nitrosation sites in four hydrogenase proteins predicted using the iSNO-PseAAC prediction tool (http://app.aporc.org/iSNO-PseAAC/) [35]. Arabidopsis: ferredoxin hydrogenase (Arabidopsis thaliana) NP_567496.4; Medicago: protein NAR1 (Medicago truncatula) XP_003606579.2; Artemisia: ferredoxin hydrogenase (Artemisia annua) PWA71961.1; Human: cytosolic iron-sulfur assembly component 3 isoform 1 (Homo sapiens) NP_071938.1. Alignment table signifies that the regions are aligned in the amino acid sequences (highlighted in Figure 1). Target cysteine residues which may become –SNO are highlighted here in red. –SNO: nitrosated thiol.
|
Arabidopsis |
Medicago |
Artemisia |
Human |
||||
|
Position of -SNO |
Sequence |
Position of -SNO |
Sequence |
Position of -SNO |
Sequence |
Position of -SNO |
Sequence |
|
24 |
LNDFIAPSQACVISLKDSKPI |
24 |
VNDFIVPSQACTVSLKERRLK |
24 |
LNDYIAPSQGCVVSMKSGSDR |
|
|
|
64 |
VKISLKDCLACSGCITSAETV |
|
|
68 |
VKISLKDCLACSGCITSAETV |
|
|
|
|
|
|
|
|
|
179 |
FVRRFRGQADCRQALPLLASA |
|
177 |
QSPLPVLSSACPGWICYAEKQ |
176 |
KSSLPMISSACPGLICYAEKS |
181 |
TSSLPMISSACPGWICYAEKT |
190 |
RQALPLLASACPGWICYAEKT |
|
182 |
VLSSACPGWICYAEKQLGSYV |
|
|
186 |
MISSACPGWICYAEKTLGSYV |
195 |
LLASACPGWICYAEKTHGSFI |
|
233 |
HEVYHVTVMPCYDKKLEAARD |
232 |
EEVYHVTVMPCYDKKLEASRD |
237 |
GDIYHVTVMPCYDKKLEASRD |
246 |
DKIYHVTVMPCYDKKLEASRP |
|
|
|
|
|
|
|
270 |
NQEHQTRDVDCVLTTGEVFRL |
|
362 |
EGKTVLKFALCYGFQNLQNIV |
366 |
DGETVLKFALCYGFSNLQKNI |
365 |
EGKTVLKFALCYGFRNLQNVV |
|
|
|
380 |
NIVRRVKTRKCDYQYVEIMAC |
|
|
|
|
385 |
NLVQRLKRGRCPYHYVEVMAC |
|
394 |
YVEIMACPAGCLNGGGQIKPK |
|
|
|
|
|
|
|
|
|
|
|
|
|
453 |
THWLQGTDSECAGRLLHTQYH |
The three-dimensional orientation of amino acids may be important for S-persulfidation of these proteins as well [40], although there appears to be no evidence in the literature of hydrogenases being covalently modified in this way. This being said, it is known that hydrogenases are inhibited by H2S [51], perhaps by attack on the metal center, as suggested for NO [38].
All three sequences (Human, Arabidopsis, and Artemisia: Figure 1) have eight conserved tyrosine residues. It is possible that they may be used for nitro-tyrosine formation, but there is no evidence of the conserved region used by Urmey and Zondlo [41]. In a similar way, looking for the cysteine residues which may be glutathionylated [43], there is no evidence of this sequence being in the hydrogenase amino acid sequences investigated here (Figure 1). From what can be considered a rather naïve point of view, using bioinformatics, it is therefore possible that hydrogenases in higher organisms are inhibited by NO through thiol modification, and possibly by ROS, but as yet there is little evidence that such control is significant.
3. Conclusions and Perspectives
There is a growing body of evidence that molecular hydrogen is perceived by organisms and has beneficial effects. Lower plants such as C. reinhardtii are known to produce substantial quantities of H2, so much so that their use as a source of H2 to be used as a biofuel has been suggested [30,31]. Higher plants also contain hydrogenases, which catalyze the reversible oxidation of H2. H2 is likely to be present in cells spatially and temporally with other reactive molecules used in cell signalling, such as ROS, NO, and H2S. Therefore, the interplay between H2 enzymes and metabolism will need to take this into account in future enquiries. Spatial and temporal measurements of all the reactive molecules involved in signalling need to be undertaken, probably requiring new fluorescent probes. Only by knowing where and when all relevant players in the regulatory orchestra are accumulated, will a full understanding of the manner in which they give a coordinated response be gained.
Hydrogenase enzymes are known to be inhibited by NO [32]. Even though –SNO formation sites can be predicted in hydrogenase sequences using algorithms such as iSNO-PseAAC [32], there is little, if any, experimental evidence that they are used in such a way, rather that the metal centres are likely targets [38]. Similarly, hydrogenases are known to be inhibited by H2S [52], but again there is little evidence of such proteins being S-persulfidated. Future experimental work should be focused on clarifying if there are any effects of NO, ROS, and H2S on the accumulation of H2 in plant cells.
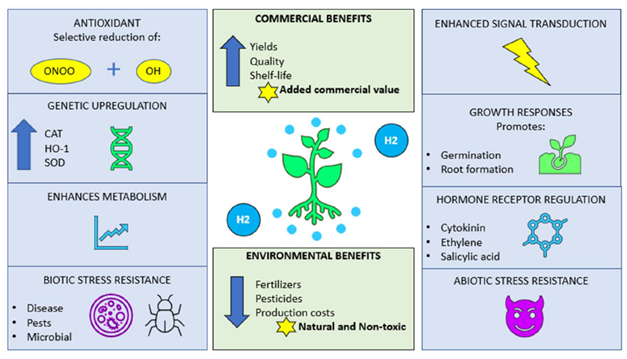
The bioavailability of H2 for a plant will also be influenced by the environment, perhaps by associated organisms. However, it is now well known that climate change-induced abiotic stress events such as droughts, floods, or soil infertility in particular are exerting a negative influence on agricultural yields. With the frequency of such challenging situations increasing, there is an inescapable need to search for yield enhancing strategies to meet the target of food security for feeding the growing human population. Another aspect that favours the utilization of non-toxic substances such as molecular hydrogen, is the current and continued usage of chemical fertilizers for enhancing yields. These practices are not sustainable when considering long-term future plans as the chemicals used in these products are recognized as being environmentally destructive, on land and in aqueous regions, where there is a threat to life caused by noxious chemicals leaching from surrounding farm lands. Figure 2 gives a brief synopsis of how molecular hydrogen can effectively increase the quality, yields, and longevity of produce whilst reducing both production and environmental costs.
Figure 2. Possible mechanisms by which H2 acts in as a protective agent within plant systems. Several ways are shown by which H2 may act, whilst highlighting the benefits for both agricultural and environmental sustainability. ONOO: peroxynitrite; OH: hydroxyl radical; CAT: catalase; HO-1; haem oxygenase-1; and SOD: superoxide dismutase.
Of particular interest to plant science is how the application of H2 can be carried out in a commercial setting [20], and how this may be made sustainable and safe, considering hydrogen is highly inflammable. Spray treatments with HRW are probably the most convenient, cost-effective, and practical methods, where delivery can theoretically be applied either onto the soil or directly onto the foliage. In light of this, and with future vertical farming in mind, future inquiry should also include the application of H2 within hydroponic cultures.
It is extremely unlikely that plant cells perceive the presence of molecular hydrogen in a truly classical manner, by utilizing a cell receptor protein. Effects of H2 application have been seen on the levels of some reactive signalling molecules, particularly hydroxyl radicals and peroxynitrite, with other ROS and RNS being relatively unaffected [14]. Effects have also been reported on haem-oxygenase activity [76], whilst it has been postulated that the physical properties of H2 may be important in mediating HO-1 activity [16]. Certainly, much more work needs to be carried out to ascertain how H2 has effects on the biochemical processes inside cells. Many enzymes and regulatory proteins are redox sensitive, and the manner in which H2 interacts with the intracellular redox environment will need to be explored. Therefore, future work may also need to focus on exploring how H2 alters gene expression and the complement of proteins in cells. This may, of course, be different in distinct plant tissues, so work would need to be carried out on roots and leaves, for example, as well as using specialist cells within those tissues, such as guard cells.
It is reasonably well established that H2 has profound effects on plants, and can promote plant growth and development, and help to alleviate stress responses. Unlike ROS, NO, and H2S, which are all extremely toxic, (despite being used as signalling molecules) [13,86], molecular hydrogen, either as a gas or dissolved in water (HRW), is thought to be biologically safe [85]. Therefore, manipulation of the availability of molecular hydrogen to plants, and a full appreciation of the effects it elicits, along with an understanding of the underpinning mechanisms of action of H2, should be a priority in future plant science endeavours for such work is likely to enhance plant growth and crop yields in the future.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][33][34][29][30][31][32][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82]
This entry is adapted from the peer-reviewed paper 10.3390/plants9091136
References
- [1] Huang, L. Molecular hydrogen: a therapeutic antioxidant and beyond. Med. Gas Res., 2016, 6, 219–222.
- [2] Li, C., Gong, T., Bian, B., Liao, W. Roles of hydrogen gas in plants: a review. Functional Plant Biology, 2018, 45, 783-792.
- [3] Ge, L., Yang, M., Yang, N.-N., Yin, X.-X., Song, W.-G. Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget., 2017, 8, 102653–102673.
- [4] Hong, Y., Chen, S., Zhang, J.M. Hydrogen as a selective antioxidant: A review of clinical and experimental studies. Journal of International Medical Research, 2010, 38, 1893-1903.
- [5] Zeng, J., Ye, Z., Sun, X. Progress in the study of biological effects of hydrogen on higher plants and its promising application in agriculture. Med. Gas Res., 2014, 4, 15.
- [6] Miller, G., Suzuki, N., Ciftci-Yilmaz, S., Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell and Environment, 2010, 33, 453-467.
- [7] Corpas, F.J. Reactive nitrogen species (RNS) in plants under physiological and adverse environmental conditions: current view. Progress in Botany, 2016, 78, 97-119.
- [8] Filipovic, M.R., Jovanović, V.M. More than just an intermediate: hydrogen sulfide signalling in plants. Journal of Experimental Botany, 2017, 68, 4733–4736.
- [9] Lisjak, M., Teklic, T., Wilson, I.D., Whiteman, M., Hancock, J.T. Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell and Environment, 2013, 36, 1607-1616.
- [10] Hancock, J.T., Whiteman, M. Hydrogen sulfide and cell signaling: team player or referee? Plant Physiology and Biochemistry, 2014, 78, 37-42.
- [11] Sánchez-Vicente, I., Fernández-Espinosa, M.G., Lorenzo, O. Nitric oxide molecular targets: reprogramming plant development upon stress. J. Exp. Bot., 2019, 70, 4441–4460.
- [12] Ohsawa, I., Ishikawa, M., Takahashi, K., Watanabe, M., Nishimaki, K., Yamagata, K., Katsura, K., Katayama, Y., Asoh, S. and Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine, 2007, 13, 688.
- [13] Lin, Y., Zhang, W., Qi, F., Cui, W., Xie, Y., Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J. Plant Physiol., 2014, 171, 1-8.
- [14] Hancock, J.T., Hancock, T.H. Hydrogen gas, ROS metabolism, and cell signaling: Are hydrogen spin states important? Reactive Oxygen Species, 2018, 6, 389–395.
- [15] Vignais, P.M., Billoud, B., Mayer, J. Classification and phylogeny of hydrogenases. FEMS Microbiology Reviews, 2001, 25, 455–501.
- [16] Hylemon, P.B., Harris, S.C., Ridlon, J.M. Metabolism of hydrogen gases and bile acids in the gut microbiome. FEBS Letters, 2018, 592, 2070-2082.
- [17] Ostojic, S.M. Non-gut microbiota as a source of bioactive hydrogen. Postgraduate Medical Journal, 2017, 93,170.
- [18] Jeanneret, R., Contino, M., Polin, M. A brief introduction to the model microswimmer Chlamydomonas reinhardtii. The European Physical Journal Special Topics, 2016, 225, 2141–2156.
- [19] Hemschemeier, A., Fouchard, S., Cournac, L., Peltier, G., Happe, T. Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks. Planta, 2008, 227, 397–407.
- [20] Meuser, J.E., D’Adamo, S., Jinkerson, R.E., Mus, F., Yang, W., Ghirardi, M.L., Seibert, M, Grossman A.R., Posewitz, M.C. Genetic disruption of both Chlamydomonas reinhardtii [FeFe]-hydrogenases: Insight into the role of HYDA2 in H2 production. Biochemical and Biophysical Research Communications, 2012, 417, 704-709.
- [21] Happe, T., Kaminski, A. (2002) Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem., 2002, 269, 1022–1032.
- [22] Melis, A., Zhang, L., Forestier, M., Ghirardi, M.L., Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiolology, 2000, 122, 127–135.
- [23] Forestier, M., King, P., Zhang, L., Posewitz, M., Schwarzer, S. Happe, T., Ghirardi, M.L., Seibert, M. Expression of two [Fe]-hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. European Journal of Biochemistry, 2003, 270, 2750-2758.
- [24] Fouchard, S., Hemschemeier, A., Caruana, A., Pruvost, J., Legrand, J., Happe, T., Peltier, G., Cournac, L. (2005) Autotrophic and mixotrophic hydrogen photoproduction in sulfur-deprived Chlamydomonas cells. Appl. Environ. Microbiol. 2005, 71, 6199–6205.
- [25] Torzillo, G., Scoma, A., Faraloni, C., Ena, A., Johanningmeier, U. Increased hydrogen photoproduction by means of a sulfur-deprived Chlamydomonas reinhardtii D1 protein mutant. International Journal of Hydrogen Energy, 2009, 34, 4529-4536.
- [26] Philipps, G., Happe, T., Hemschemeier, A. Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta, 2012, 235, 729–745.
- [27] Khetkorn, W., Rastogi, R.P., Incharoensakdi, A., Lindblad, P., Madamwar, D., Pandey, A., Larroche, C. Microalgal hydrogen production – A review. Bioresour Technol., 2017, 243, 1194-1206.
- [28] Mahidhara, G., Burrow, H., Sasikala, Ch., Ramana, Ch.V. Biological hydrogen production: molecular and electrolytic perspectives. World J. Microbiol. Biotechnol., 2019, 35, 116.
- [29] Krasna, A.I., Rittenberg, D. The inhibition of hydrogenase by nitric oxide. Proc. Natl. Acad. Sci. U.S.A., 1954, 40, 225-227.
- [30] Feng, J., Chen, L., Zuo, J. Protein S-nitrosylation in plants: Current progresses and challenges. Journal of Integrative Plant Biology, 2019, 61, 1206-1223.
- [31] Kolbert, Z., Feigl, G., Bordé, Á., Molnár, Á., Erdei, L. Protein tyrosine nitration in plants: Present knowledge, computational prediction and future perspectives. Plant Physiology and Biochemistry, 2017, 113, 56-63.
- [32] Xu, Y.; Ding, J.; Wu, L.Y.; Chou, K.C. iSNO-PseAAC: Predict cysteine S-nitrosylation sites in proteins by incorporating position specific amino acid propensity into pseudo amino acid composition. PLoS ONE 2013, 8, e55844.
- [33] Ascenzi, P.; Colasanti, M.; Persichini, T.; Muolo, M.; Polticelli, F.; Venturini, G.; Bordo, D.; Bolognesi, M. Re-evaluation of amino acid sequence and structural consensus rules for cysteine-nitric oxide reactivity. Biological Chemistry, 2000, 381, 623–627.
- [34] Jia J., Arif A., Terenzi F., Willard B., Plow E.F., Hazen S.L., Fox P.L. Target-selective protein S-nitrosylation by sequence motif recognition. Cell, 2014, 159, 623-634.
- [35] Ceccaldi, P., Etienne, E., Dementin, S., Guigliarelli, B., Léger, C., Burlat, B. Mechanism of inhibition of NiFe hydrogenase by nitric oxide. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 2016, 1857, 454-461.
- [36] Aroca, A., Benito, J.M., Gotor, C., Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. Journal of Experimental Botany, 2017, 68, 4915-4927.
- [37] Ju, Y., Fu, M., Stokes, E., Wu, L., Yang, G. H₂S-Mediated protein S-sulfhydration: A prediction for its formation and regulation. Molecules, 2017, 22, 1334.
- [38] Urmey, A.R., Zondlo, N.J. Design of a protein motif responsive to tyrosine nitration and an encoded turn-off sensor of tyrosine nitration. Biochemistry, 2019, 58, 2822-2833.
- [39] Sigrist, C.J.A., de Castro, E, Cerutti, L., Cuche, B.A., Hulo, N, Bridge, A., Bougueleret, L., Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Research, 2012, doi: 10.1093/nar/gks1067
- [40] Scheuermann, M.J., Forber, C.R., Zondlo, N.J. Redox-responsive protein design: design of a small protein motif dependent on glutathionylation. Biochemistry, 2018, 57, 6956-6963.
- [41] Aldon, D., Mbengue, M., Mazars, C., Galaud, J.-P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci., 2018, 19, 665.
- [42] Renwick, G.M., Giumarro, C., Siegel, S.M. Hydrogen metabolism in higher plants. Plant Physiology, 1964, 39, 303–306.
- [43] Mondy, S., Lenglet, A., Cosson, V., Pelletier, S., Pateyron, S., Gilard, F., Scholte, M., Brocard, L., Couzigou, J.-M., Tcherkez, G., Péan, M., Ratet, P. GOLLUM [FeFe]-hydrogenase-like proteins are essential for plant development in normoxic conditions and modulate energy metabolism. Plant Cell and Environment, 2014, 37, 54-69.
- [44] Nakamura, M., Buzas, D.M., Kato, A., Fujia, M., Kurata, N., Kinoshita, T. The role of Arabidopsis thaliana NAR1, a cytosolic iron-sulfur cluster assembly component, in gametophytic gene expression and oxidative stress responses in vegetative tissue. New Phytologist, 2013, 199, 925-935.
- [45] Scholte, M., d'Erfurth, I., Rippa, S., Mondy, S., Cosson, V., Durand, P., Breda, C., Trinh, H., Rodriguez-Llorente, I., Kondorosi, E., Schultze, M., Kondorosi, A., Ratet, P. (2002) T‐DNA tagging in the model legume Medicago truncatula allows efficient gene discovery. Molecular Breeding, 2002, 10,203–215.
- [46] Luo, D., Bernard, D.G., Balk, J., Hai, H., Cui X. The DUF59 family gene AE7 acts in the cytosolic iron‐sulfur cluster assembly pathway to maintain nuclear genome integrity in Arabidopsis. The Plant Cell, 2012, 24, 4135–4148.
- [47] Sievers, F.., Wilm, A., Dineen, D., Gibson, T.J., Karplus, K., Li, W., Lopez, R., McWilliam, H., Remmert, M., Söding, J., Thompson, J.D., Higgins D.G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol., 2011, 7, 539.
- [48] Vincent, K.A., Belsey, N.A., Lubitz, W., Armstrong, F.A. Rapid and reversible reactions of [NiFe]-hydrogenases with sulfide. J. Am. Chem. Soc., 2006, 128, 7448-7449.
- [49] Irvine, P., Smith, M. and Dong, Z. (2004). Hydrogen fertilizer: bacteria or fungi? Acta Hortic., 2004, 631, 239-242.
- [50] Suzuki, A., Ito, M., Hamaguchi, T., Mori, H., Takeda, Y., Baba, R., Watanabe, T., Kurokawa, K., Asakawa, S., Hirayama, M., Ohno, K. Quantification of hydrogen production by intestinal bacteria that are specifically dysregulated in Parkinson's disease. PLoS One, 2018, 13, e0208313.
- [51] Ivarsson, M., Schnürer, A., Bengtson, S., Neubeck, A. Anaerobic fungi: A potential source of biological H2 in the oceanic crust. Frontiers in Microbiology, 2016, 7, 674.
- [52] Maimaiti, J., Zhang, Y., Yang, J., Cen, Y.P., Layzell, D.B., Peoples, M., Dong, Z. Isolation and characterization of hydrogen-oxidizing bacteria induced following exposure of soil to hydrogen gas and their impact on plant growth. Environ. Microbiol., 2007, 9, 435–444.
- [53] Wilson, P.W., Umbreit, W.W. Mechanism of symbiotic nitrogen fixation. III. Hydrogen as a specific inhibitor. Arch. Mikrobiol., 1937, 8, 44-57.
- [54] Schubert, K.R., Evans, H. Hydrogen evolution: a major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc. Natl. Acad. Sci. USA, 1976, 73, 1207–1211.
- [55] Ruiz-Argüeso, T., Maier, R.J., Evans, H.J. Hydrogen evolution from alfalfa and clover nodules and hydrogen uptake by free-living Rhizobium meliloti. Appl. Environ. Microbiol., 1979, 37, 582–587.
- [56] Golding, A., Dong, Z. Hydrogen production by nitrogenase as a potential crop rotation benefit. Environ Chem Lett, 2010, 8, 101–121.
- [57] Kanno, M., Constant, P., Tamaki, H., Kamagata, Y. Detection and isolation of plant-associated bacteria scavenging atmospheric molecular hydrogen. Environ. Microbiol., 2016, 18, 2495-2506.
- [58] Tibelius, K.H. and Knowles. Hydrogenase activity in Azospirillum brasilense is inhibited by nitrite, nitric oxide, carbon monoxide, and acetylene. Journal of bacteriology, 1984, 160, (1),.103-106.
- [59] Ahmed, A., Lewis, R.S. Fermentation of biomass-generated synthesis gas: effects of nitric oxide. Biotechnol. Bioeng., 2007, 97, 1080-1086.
- [60] Kolbert, Zs., Barroso, J.B., Brouquisse, R., Corpas, F.J., Gupta, K.J., Lindermayr, C., Loake, G.J., Palma, M., Petřivalský, M., Wendehenne, D., Hancock, J.T. A forty year journey: the generation and roles of NO in plants. Nitric Oxide, 2019, 93, 53-70.
- [61] Gotor, C., García, I., Aroca, Á., Laureano-Marín, A.M., Arenas-Alfonseca, L., Jurado-Flores, A., Moreno, I., Romero, L.C. Signaling by hydrogen sulfide and cyanide through post-translational modification. J. Exp. Bot., 2019, 70, 4251-4265.
- [62] Liu, F., Jiang, W., Han, W., Li, J., Liu, Y. Effects of hydrogen‐rich water on fitness parameters of rice plants. Agronomy Journal, 2017, 109, 2033, 2039.
- [63] http://www.molecularhydrogeninstitute.com/concentration-and-solubility-of-h2
- [64] Seo, T., Kurokawa, R., Sato, B. A convenient method for determining the concentration of hydrogen in water: use of methylene blue with colloidal platinum. Med. Gas Res., 2012, 2, 1.
- [65] Zhai, Y., Zhou, X., Dai, Q., Fan, Y., Huang, X. Hydrogen-rich saline ameliorates lung injury associated with cecal ligation and puncture-induced sepsis in rats. Exp Mol Pathol., 2015, 98, 268-276.
- [66] Da-Silva, C.J., Modolo, L.V. Hydrogen sulfide: a new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot. Bras., 2018, 32, no.1.
- [67] Hancock, J.T. Methods for the addition of redox compounds. In: J.T. Hancock, & M.E. Conway (Eds.), Redox-Mediated Signal Transduction, 2019, pp. 13-25. Humana, New York, NY.
- [68] Sanadze, G.A. Absorption of molecular hydrogen by green leaves in light. Fiziol Rast, 1961, 8, 555-559.
- [69] Cao, Z., Duan, X., Yao, P., Cui, W., Cheng, D., Zhang, J., Jin, Q., Chen, J., Dai, T., Shen., W. Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int. J. Mol. Sci., 2017, 18, 2084.
- [70] Lin, Y., Zhang, W., Qi, F., Cui, W., Xie, Y., Shen, W. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. Journal of Plant Physiology, 2014, 171, 1–8.
- [71] Hu, H., Li, P., Wang, Y., Gu, R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chemistry, 156, 100-109.
- [72] Chen, M., Cui, W., Zhu, K., Xie, Y., Zhang, C., Shen, W. Hydrogen-rich water alleviates aluminium-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J. Hazard. Mater., 2014, 267, 40–47.
- [73] Xu, D., Cao, H., Fang, W., Pan, J., Chen, J., Zhang, J., Shen, W. Linking hydrogen-enhanced rice aluminum tolerance with the reestablishment of GA/ABA balance and miRNA-modulated gene expression: A case study on germination. Ecotoxicol. Environ. Saf., 2017, 145, 303–312.
- [74] Zeng, J., Zhang, M., Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress response in plants. PLoS ONE, 2013, 8, e71038.
- [75] Xie, Y., Mao, Y., Lai, D., Zhang, W., Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE, 2012, 7, e49800.
- [76] Xu, S., Zhu, S.S., Long, J.Y., Wang, N., Wang, R., Shen W. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil, 2013, 370, 47–57.
- [77] Cui, W., Gao, C., Fang, P., Lin, G., Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater., 2013, 260, 715–724.
- [78] Cui, W., Fang, P., Zhu, K., Mao, Yu., Gao, C., Xie, Y., Wang, J., Shen, W.B. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol. Environ. Saf., 2014, 5, 103–111.
- [79] Fryzova, R., Pohanka, M., Martinkova, P., Cihlarova, H., Brtnicky, M., Hladky, J., Kynicky, J. Oxidative stress and heavy metals in plants. Rev. Environ. Contam. Toxicol., 2018, 245, 129-156.
- [80] Jin, Q., Zhu, K., Cui, W., Xie, Y., Han, B., Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell and Environment, 2013, 36, 956–969.
- [81] LeBaron, T.W., Kura, B., Kalocayova, B., Tribulova, N., Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules, 2019, 24, 2076.
- [82] Hancock, J.T. Harnessing evolutionary toxins for signaling: reactive oxygen species, nitric oxide and hydrogen sulfide in plant cell regulation. Frontiers in Plant Science, 2017, 8, 189.