Besides traditional risk factors, accumulated evidence suggested that a high inflammatory burden has emerged as a key characteristic modulating both the pathogenesis and progression of cardiovascular diseases, inclusive of atherosclerosis and myocardial infarction. To mechanistically elucidate the correlation, signalling pathways downstream to Toll-like receptors, nucleotide oligomerisation domain-like receptors, interleukins, tumour necrosis factor, and corresponding cytokines were raised as central mechanisms exerting the effect of inflammation. Other remarkable adjuvant factors include oxidative stress and secondary ferroptosis. These molecular discoveries have propelled pharmaceutical advancements. Statin was suggested to confer cardiovascular benefits not only by lowering cholesterol levels but also by attenuating inflammation. Colchicine was repurposed as an immunomodulator co-administered with coronary intervention. Novel interleukin-1β and −6 antagonists exhibited promising cardiac benefits in the recent trials as well. Moreover, manipulation of gut microbiota and associated metabolites was addressed to antagonise inflammation-related cardiovascular pathophysiology. The gut-cardio-renal axis was therein established to explain the mutual interrelationship.
1. Background
Inflammation remains a long-standing clinical challenge. An elevated inflammatory status has recently been proposed as an independent risk factor for developing end-organ comorbidities and unfavourable prognosis of various chronic illnesses [
1]. Cardiovascular diseases were among those entities proposed to be closely intertwined with inflammation. The heavy clinical burden due to high incidence, leading cause of morbidity, and suboptimal, albeit significantly improved, management of cardiovascular diseases have prompted the development of novel modalities to treat this population. Management against residual inflammatory burden after an index event has gradually been recognised as pivotal to prevent systemic sequelae and improve holistic care. Early meta-analysis in the last decade demonstrated that elevated C-reactive protein (CRP) levels were positively correlated with the risk of coronary artery disease (CAD), ischaemic stroke, and mortality in individuals without underlying vascular diseases [
2]. Inflammation was further addressed, giving rise to CAD through its correlation with plaque rupture and thromboembolism [
3]. As for terminal consequences, decompensatory remodeling secondary to inflammatory heart leads to heart failure with reduced ejection fraction [
4]. Accordingly, multiple clinical trials examined potential strategies to ameliorate inflammation; however, their efficacies remain debatable. Besides anti-inflammatory medications, manipulation of the gut microbiota and its metabolites emerged as novel therapeutic measures.
2. Inflammation and Cardiovascular Diseases
2.1. Atherosclerosis
Inflammation has been reported to predispose individuals to atherosclerosis [
5]. Activated endothelial cells promote the expression of inflammatory markers. Lymphocytes and monocytes migrate toward the endothelium and further infiltrate the arterial wall to induce atherogenesis [
6]. The healing of vascular injury depends on the activation of the inflammatory signal, which is the substrate for the formation of atherosclerotic plaques. Historically, this vulnerable plaque theory was conceptualised to link inflammation to vascular events. Toll-like receptor (TLR) 4, nuclear factor κ-light-chain-enhancer of activated B cells (NFκB), and Janus kinase/signal transducer and activator of transcription are inflammation-related pathways that mediate atherosclerosis [
7]. The inflammation further facilitates erosion and potential rupturing of the local plaque, resulting in thromboembolic events. From a preventive medicine perspective, amelioration of the inflammatory status has been used to reduce the risk of adverse cardiovascular outcomes and atherothrombosis.
2.2. Myocardial Infarction
Inflammation after myocardial infarction (MI) is another issue of concern. A previous transcriptome study longitudinally mapped the polarisation of local macrophages [
8]. On the first day post MI, initiation of interleukin (IL)-1 signalling, as well as the recognition of damage-associated molecular patterns, was shown to lead to the breakdown of extracellular matrix (ECM) by released matrix metalloproteinase (MMP). Elevated oxidative stress has been proposed to cause cardiovascular damage. In conjunction, reprogrammed metabolism subsequently promoted phagocytosis by resident macrophages. Eventually, transduction of integrin, transforming growth factor β (TGFβ) receptor 1, IL-4 receptor α chain signalling, and signal transducer and activator of transcription 3 signalling resulted in the release of collagen ECM repair factors after 1 week. Neutrophils also participate in the post-MI inflammatory network [
9]. AMP-activated protein kinase, NF-κB, tumour necrosis factor (TNF)-α, and calcium signalling were identified as the first responders to diapedesis, followed by the activation of cathepsin protease for apoptosis [
10]. These also resulted in left ventricular (LV) wall thickening and hypertrophy. ECM reorganisation and scar formation are known consequences of the inflammation cascade [
11].
3. Molecular Mechanism Underlying Inflammation
The molecular mechanism governing the role of inflammation in cardiovascular diseases has garnered considerable research attention. The inflammatory status can be activated by diverse aetiologies, such as myocardial ischaemia, viral myocarditis, hypertrophic or autoimmune cardiomyopathy, and genetic diseases. The injured myocardium triggers both innate and adaptive immunity [
21]. In addition, cholesterol crystals, neutrophil extracellular traps, atheroprone flow, and hypoxia contribute to the activation of the inflammatory status. Binding of pathogen-associated molecular patterns or damage-associated molecular patterns to pattern recognition receptors, including TLRs and nucleotide oligomerisation domain (NOD)-like receptors, on local cardiomyocytes or residing immune cells, induces downstream signal transduction pathways. Eventually, the cascade leads to the upregulation of chemokines and inflammatory cytokines. The most commonly involved players are the IL family and TNF. Transcriptional profiling has also reported that the genetic landscape associated with inflammation is altered in patients with heart failure and myocardial ischaemia [
22]. In addition, macrophages and neutrophils are recruited collectively to initiate adaptive immunity.
4. Pharmaceutical Advancements
4.1. Colchicine
To achieve adequate anti-inflammation, the indication for colchicine was revisited. As a traditional and orally available medication against the NOD-like receptor protein 3 (NLRP3) inflammasome, the use of colchicine has been broadened, despite notorious gastrointestinal adverse effects. Further trials with colchicine were undertaken to evaluate cardiovascular impacts in various clinical settings. In the Colchicine Cardiovascular Outcomes trials (COLCOT) [
39] study with 4745 participants sustaining recent MI within one month, the introduction of low-dose colchicine was associated with a significant reduction in subsequent ischaemic cardiovascular events. Based on this finding, in the Low-Dose Colchicine 2 (LoDoCo2) trial [
40], 5522 patients with chronic CAD were included to receive colchicine (0.5 mg) per day or placebo. At the 28.6-month follow-up, the composite of MACE was remarkably attenuated following the administration of anti-inflammatory agents. Additionally, multiple attempts have been made to delineate the cardiovascular benefit by ameliorating periprocedural vascular injury and inflammation secondary to percutaneous coronary intervention (PCI). The inflammatory risk in patients undergoing PCI is clinically relevant. A large retrospective study assessing 7026 subjects after intervention proposed that persistently high CRP levels were significantly correlated with all-cause mortality and MI risk [
41].
4.2. Interleukin-1 Antagonist
Meanwhile, other anti-inflammatory agents, apart from colchicine, were assessed for their cardiovascular effects. In the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) trail [
47], 10,061 post-MI subjects with elevated CRP were enrolled. Targeting IL-1β with canakinumab markedly reduced the rate of recurrent cardiovascular events. Methotrexate, a widely used anti-inflammatory agent in rheumatological diseases, was evaluated but failed to improve the clinical efficacy of secondary prevention in patients with stable atherosclerosis. In the Cardiovascular Inflammation Reduction Trial (CIRT) [
48], 4786 individuals with previous MI or multivessel coronary disease, concomitant diabetes mellitus (DM), or metabolic syndrome were enrolled. At the 2.3-year follow-up, methotrexate did not reduce the composite rate of cardiovascular mortality, nonfatal MI, or nonfatal stroke. Anakinra-mediated IL-1 blockade has been evaluated for its clinical efficacy as a potential therapeutic target. Abbate et al. conducted a pilot study with 30 patients sustaining STEMI [
49]. The first dose of anakinra 100 mg was administered within 24 h of primary PCI and continued for 14 days. A significant decrease in serum CRP level and the rate of mortality or new-onset heart failure was documented, with no reduction in the level of CK-MB and MACE incidence.
5. The Role of Gut Microbiota
A disorganised profile of the intestinal microorganism was proposed to be interrelated with inflammation under the context of cardiovascular diseases [
60]. Amelioration of inflammatory status by modulating the gut microbiota composition is expected to serve as a new modality. Translocation of the microorganisms and shedding of the bacterial wall compound can contribute to the inflammatory status. Additionally, not only dysbiosis but also the altered metabolism gives rise to inflammation. First, the short-chain fatty acid generated by anaerobic fermentation of fibre is considered pivotal for initiating inflammatory signalling [
61]. Second, butyrate is another end metabolite of fermentation that had been identified to govern regulatory T cells for orchestrating inflammation and recognised prerequisite to maintain the intestinal barrier [
62]. A recent study with metagenome sequencing indicated the expression of the butyrate-encoding gene was negatively correlated with the level of inflammatory markers, which was proposed secondary to the altered abundance of
Roseburia and
Eubacterium in subjects with carotid atherosclerosis [
63]. Third, lipopolysaccharide (LPS) shed from Gram-negative bacteria was also pointed out as the bridge of remodeled microorganisms to inflammation and eventually attributing to the pathogenesis of cardiovascular diseases. The enteric LPS invades systemic circulation and ignites inflammation after being recognised by TLRs. The elevated endotoxin level and pro-inflammatory status eventually compromise cardiac and vascular function [
64].
Moreover, trimethylamine N-oxide (TMAO) was identified as another chief transmitter from dysbiosis to the inflammatory status impacting cardiovascular manifestations based on the metabolomic perspective. The quantity of TMAO was determined by age, body mass index, and especially the gut microbiome composition.
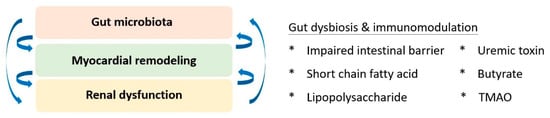
6. Gut-Cardio-Renal Triplet
Based on the accumulating evidence of mutual interplay, the triplet of the gut-cardio-renal axis was conceptualised to modify traditional cardiorenal syndrome (
Figure 2). The homeostasis of gut microbiota and its immunoregulatory effects are pivotal for maintaining the physiology of both the cardiac and renal systems. Regarding kidney physiology, impaired intestinal barrier function, microbial dysbiosis, compromised immunity, and toxin production were primary factors that correlated with altered gut microbiota and renal dysfunction [
87]. Short-chain fatty acids have been identified as key players in immune regulation, signalling of G-protein-coupled receptors, and antagonists of histone deacetylases for epigenetic modulation [
88]. Additionally, the pathogenesis of cardiorenal dysfunction implicates the effect of uraemic toxins, including indoxyl sulfate, p-cresyl sulfate, p-cresol, phenylacetic acid, indole-3-acetic acid, homocysteine, hippuric acid, and phenol [
89]. Collectively, the search for an upstream therapeutic target for better-integrated care remains a priority. Linaclotide, a guanylate cyclase C agonist, was identified as a potential candidate. Low-dose linaclotide was suggested to prevent and manage cardiorenal syndrome by downregulating plasma TMAO, uraemic toxin, and colonic claudin-1 levels [
90].
Figure 2. The gut-cardio-renal triplet. The gut microbiome and the pathophysiology of cardiac and renal systems are mutually interactive and dependent on the inflammatory response. TMAO: trimethylamine-N-oxide.
7. Conclusions
In conclusion, the residual inflammatory burden indicates a novel phenotype in patients with cardiovascular disease. In addition to traditional cardiovascular risk factors, the status of inflammation has been established to develop cardiovascular dysfunction, as well as compromise prognosis. With advances in our understanding of molecular networks, the generation of next-generation immunomodulatory agents specifically targeting new therapeutic targets identified via molecular investigations remains the current priority. Mechanism-based rationale to comprehend inflammation in the context of concomitant cardiovascular disease is currently the mainstream in bench studies. A better depiction of the interplay between inflammatory status and target organs constructed the gut-cardio-renal triplet. On the other end of the spectrum, future clinical trials with extended ethical backgrounds will facilitate the generalisation of these medications to orchestrate the inflammatory status from a clinical bedside perspective. Artificial intelligence with machine learning will be a novel modality for data mining and to assess the metabolome, transcriptome, and proteome. Specific management of signalling molecules will pave the way for individualised prescription and precision medicine.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020804