Medulloblastoma, the most common embryonal tumor in children, can also arise in older patients. The sonic hedgehog (SHH) pathway is altered in a significant proportion of older patients with medulloblastoma. The Alliance for Clinical Trials in Oncology cooperative group is developing the AMBUSH trial: Comprehensive Management of Adolescent and Young Adult (AYA) and Adult Patients with Medulloblastoma or Pineal Embryonal Tumors With A Randomized Placebo Controlled Phase II Focusing on Sonic Hedgehog Pathway Inhibition in SHH Subgroup Patients (Adult & Adolescent MedulloBlastoma Using Sonic Hedgehog Trial). The trial gives treatment directions for all patients and randomizes patients with average risk SHH-activated medulloblastoma to maintenance sonidegib, a hedgehog signaling pathway inhibitor, or placebo.
- medulloblastoma
- sonic hedgehog
- sonidegib
- pineal parenchymal tumor
- clinical trial
- radiotherapy
- targeted therapy
1. Introduction
2. Classification of Medulloblastoma
3. Rationale to Include Non-SHH-MB, High-Risk MB, and Pineal Tumors
4. Backbone of AMBUSH Therapeutic Strategy
4.1. Surgery
4.2. Radiotherapy
4.3. Chemotherapy
4.4. Targeted Therapy
5. Trial Design
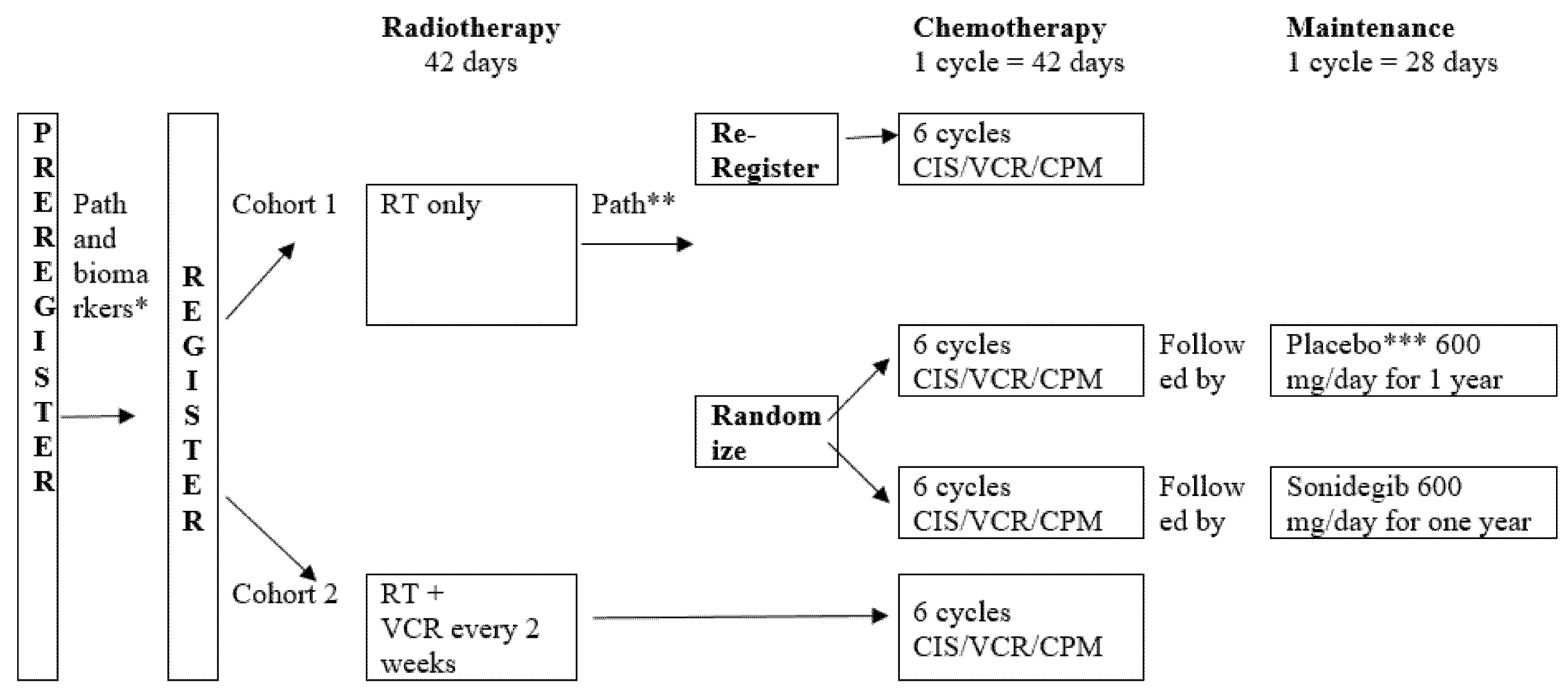
6. Objectives
7. Accrual Goal
8. Translational Research
|
Test or Observation |
Baseline |
Weekly during RT |
Prior to Chemo |
Chemo × 4 Cycles |
Pre Sonidegib/Placebo |
Monthly |
Annually Post Completions |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Approximate Wk, Wk 0 = RT start |
Wk |
Wk |
Wk |
Wk |
Wk |
Wk |
Wk 52 |
||||
|
−4 to 2 |
0 to 6 |
9 to 10 |
10 to 26 |
26 to 30 |
30 to 82 |
||||||
|
Tests and Observations |
|||||||||||
|
History and Physical |
pre RT |
x |
x |
x |
x |
each visit |
x |
||||
|
Weight (each visit) |
pre RT |
x |
x |
prn |
x |
x |
x |
||||
|
Hearing |
x |
x |
x |
y 1, 3, 5 |
|||||||
|
Vision |
x |
x |
y 1, 3, 5 |
||||||||
|
CompNeurocog |
x |
y 1, 3, 5 |
|||||||||
|
Short Neurocog |
x |
x |
x |
x |
x |
x |
x |
||||
|
QOL/PRO-CTCAE |
x |
x |
x |
x |
x |
y 1, 3, 5 |
|||||
|
Laboratory Studies |
|||||||||||
|
CBC |
pre RT |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
|
Blood Chemistries |
x |
x |
prn |
x |
x |
x |
x |
x |
x |
x |
|
|
Endocrine |
x |
x |
y 1, 3, 5 |
||||||||
|
Creatine Kinase |
x |
x |
x |
x |
x |
x |
x |
x |
x |
x |
|
|
BUN/Creatinine |
x |
x |
x (1) |
x |
x |
x |
x |
x |
x |
x |
|
|
Staging |
|||||||||||
|
Tumor Imaging |
pre RT |
x |
post cycle 2 |
x |
|||||||
|
Research Studies for Banking |
tumor, CSF, plasma |
plasma |
plasma |
||||||||
|
plasma and CSF if Recurrence |
|||||||||||
n.b. x denotes when the test or observation will be required on the protocol. Abbreviations: Wk: week; RT: radiotherapy; chemo: chemotherapy; Comp Neurocog: comprehensive neurocognitive testing; Short Neurocog: Short neurocognitive testing; QOL: quality of life; PRO: patient reported outcomes; CBC: complete blood count; BUN: blood urea nitrogen; CSF: cerebrospinal fluid; prn: as needed; y: year.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14020414
References
- Giordana, M.T.; Schiffer, P.; Lanotte, M.; Girardi, P.; Chio, A. Epidemiology of adult medulloblastoma. Int. J. Cancer 1999, 80, 689–692.
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75.
- Gajjar, A.; Chintagumpala, M.; Ashley, D.; Kellie, S.; Kun, L.E.; Merchant, T.E.; Woo, S.; Wheeler, G.; Ahern, V.; Krasin, M.J.; et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006, 7, 813–820.
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III Study of Craniospinal Radiation Therapy Followed by Adjuvant Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208.
- Von Bueren, A.O.; Kortmann, R.-D.; Von Hoff, K.; Friedrich, C.; Mynarek, M.; Müller, K.; Goschzik, T.; Mühlen, A.Z.; Gerber, N.; Warmuth-Metz, M.; et al. Treatment of Children and Adolescents With Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J. Clin. Oncol. 2016, 34, 4151–4160.
- Lannering, B.; Rutkowski, S.; Doz, F.; Pizer, B.; Gustafsson, G.; Navajas, A.; Massimino, M.; Reddingius, R.; Benesch, M.; Carrie, C.; et al. Hyperfractionated Versus Conventional Radiotherapy Followed by Chemotherapy in Standard-Risk Medulloblastoma: Results From the Randomized Multicenter HIT-SIOP PNET 4 Trial. J. Clin. Oncol. 2012, 30, 3187–3193.
- Michalski, J.M.; Janss, A.J.; Vezina, L.G.; Smith, K.S.; Billups, C.A.; Burger, P.C.; Embry, L.M.; Cullen, P.L.; Hardy, K.K.; Pomeroy, S.L.; et al. Children’s Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J. Clin. Oncol. 2021, 39, 2685–2697.
- Thomas, P.R.M.; Deutsch, M.; Kepner, J.L.; Boyett, J.M.; Krischer, J.; Aronin, P.; Albright, L.; Allen, J.; Packer, R.J.; Linggood, R.; et al. Low-Stage Medulloblastoma: Final Analysis of Trial Comparing Standard-Dose With Reduced-Dose Neuraxis Irradiation. J. Clin. Oncol. 2000, 18, 3004–3011.
- Cosman, R.; Brown, C.; De Braganca, K.; Khasraw, M. Patterns of care in adult medulloblastoma: Results of an international online survey. J. Neuro-Oncology 2014, 120, 125–129.
- Patil, R.; Gupta, T.; Maitre, M.; Dasgupta, A.; Sahay, A.; Epari, S.; Shirsat, N.; Chatterjee, A.; Krishnatry, R.; Goda, J.S.; et al. Clinical Audit of Survival Outcomes and Prognostic Factors in Adolescents and Adults with Medulloblastoma. J. Adolesc. Young-Adult Oncol. 2021.
- Penas-Prado, M.; Theeler, B.J.; Cordeiro, B.; Dunkel, I.J.; Hau, P.; Mahajan, A.; Robinson, G.W.; Willmarth, N.; Aboud, O.; Aldape, K.; et al. Proceedings of the Comprehensive Oncology Network Evaluating Rare CNS Tumors (NCI-CONNECT) Adult Medulloblastoma Workshop. Neuro-Oncology Adv. 2020, 2, vdaa097.
- Brandes, A.A.; Ermani, M.; Amista, P.; Basso, U.; Vastola, F.; Gardiman, M.; Iuzzolino, P.; Turazzi, S.; Rotilio, A.; Volpin, L.; et al. The treatment of adults with medulloblastoma: A prospective study. Int. J. Radiat. Oncol. 2003, 57, 755–761.
- Northcott, P.A.; Korshunov, A.; Pfister, S.; Taylor, M. The clinical implications of medulloblastoma subgroups. Nat. Rev. Neurol. 2012, 8, 340–351.
- Ramaswamy, V.; Taylor, M. Medulloblastoma: From Myth to Molecular. J. Clin. Oncol. 2017, 35, 2355–2363.
- Schwalbe, E.; Lindsey, J.C.; Nakjang, S.; Crosier, S.; Smith, A.J.; Hicks, D.; Rafiee, G.; Hill, R.M.; Iliasova, A.; Stone, T.; et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017, 18, 958–971.
- Sengupta, S.; Krummel, D.P.; Pomeroy, S. The evolution of medulloblastoma therapy to personalized medicine. F1000Research 2017, 6, 490.
- Herrlinger, U.; Steinbrecher, A.; Rieger, J.; Hau, P.; Kortmann, R.D.; Meyermann, R.; Schabet, M.; Bamberg, M.; Dichgans, J.; Bogdahn, U.; et al. Adult medulloblastoma: Prognostic factors and response to therapy at diagnosis and at relapse. J. Neurol. 2005, 252, 291–299.
- Takase, H.; Tanoshima, R.; Singla, N.; Nakamura, Y.; Yamamoto, T. Pineal parenchymal tumor of intermediate differentiation: A systematic review and contemporary management of 389 cases reported during the last two decades. Neurosurg Rev. 2021. online ahead of print.
- Jakacki, R.I.; Zeltzer, P.M.; Boyett, J.M.; Albright, A.L.; Allen, J.C.; Geyer, J.R.; Rorke, L.B.; Stanley, P.; Stevens, K.R.; Wisoff, J. Survival and prognostic factors following radiation and/or chemotherapy for primitive neuroectodermal tumors of the pineal region in infants and children: A report of the Childrens Cancer Group. J. Clin. Oncol. 1995, 13, 1377–1383.
- Massimino, M.; Sunyach, M.P.; Barretta, F.; Gandola, L.; Garegnani, A.; Pecori, E.; Spreafico, F.; Bonneville-Levard, A.; Meyronet, D.; Mottolese, C.; et al. Reduced-dose craniospinal irradiation is feasible for standard-risk adult medulloblastoma patients. J. Neuro-Oncology 2020, 148, 619–628.
- Paterson, E.; Farr, R.F. Cerebellar Medulloblastoma: Treatment by Irradiation of the Whole Central Nervous System. Acta Radiol. 1953, 39, 323–336.
- Friedrich, C.; von Bueren, A.O.; von Hoff, K.; Kwiecien, R.; Pietsch, T.; Warmuth-Metz, M.; Hau, P.; Deinlein, F.; Kuehl, J.; Kortmann, R.D.; et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: A prospective observational multicentre study. Eur. J. Cancer 2012, 49, 893–903.
- Majd, N.K.; Mastall, M.; Lin, H.; Dibaj, S.S.; Hess, K.R.; Yuan, Y.; Garcia, M.M.; Fuller, G.N.; Alfaro, K.D.; Gule-Monroe, M.K.; et al. Clinical characterization of adult medulloblastoma and the effect of first-line therapies on outcome; The MD Anderson Cancer Center experience. Neurooncol Adv. 2021, 3, vdab079.
- Beier, D.; Proescholdt, M.; Reinert, C.; Pietsch, T.; Jones, D.T.W.; Pfister, S.M.; Hattingen, E.; Seidel, C.; Dirven, L.; Luerding, R.; et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro-Oncology 2017, 20, 400–410.
- Von Bueren, A.O.; Friedrich, C.; Von Hoff, K.; Kwiecien, R.; Müller, K.; Pietsch, T.; Warmuth-Metz, M.; Hau, P.; Benesch, M.; Kuehl, J.; et al. Metastatic medulloblastoma in adults: Outcome of patients treated according to the HIT2000 protocol. Eur. J. Cancer 2015, 51, 2434–2443.
- Carballo, G.B.; Honorato, J.R.; De Lopes, G.P.F.; Spohr, T.C.L.D.S.E. A highlight on Sonic hedgehog pathway. Cell Commun. Signal. 2018, 16, 11.
- Kieran, M.W. Targeted treatment for sonic hedgehog-dependent medulloblastoma. Neuro-Oncology 2014, 16, 1037–1047.
