Micro/nanocarriers are organic and polymeric materials that are structurally oriented to act like capsules in aqueous and organic media in order to protect, transport, and release cargo, among other applications. Liposomes are organic micro/nanocarriers based on phospholipids that form vesicles. In contrast, polymeric micro/nanocarriers are based on amphiphilic and backbone polymers that form micelles, polymersomes, and polymeric spheres. Micro/nanocarriers are usually produced via precipitation and emulsion techniques, using a hydrophobic/hydrophilic solvent mixture, an emulsifier or surfactant, and crosslinker agents acting as template-like reaction initiators to orientate the amphiphilic backbone polymers, forming their structure. Cargo is loaded either during the assembly process, by solubilizing the cargo in the hydrophobic or hydrophilic solvent, or after the carrier’s formation, by dispersing the carriers into a high-cargo-concentration solution, followed by further cargo diffusion through the carrier.
- liposomes
- polymeric nanomicelles
- nanopolymersomes
- polymeric micro/nanospheres
- cargo delivery
1. Introduction
2. Liposomes
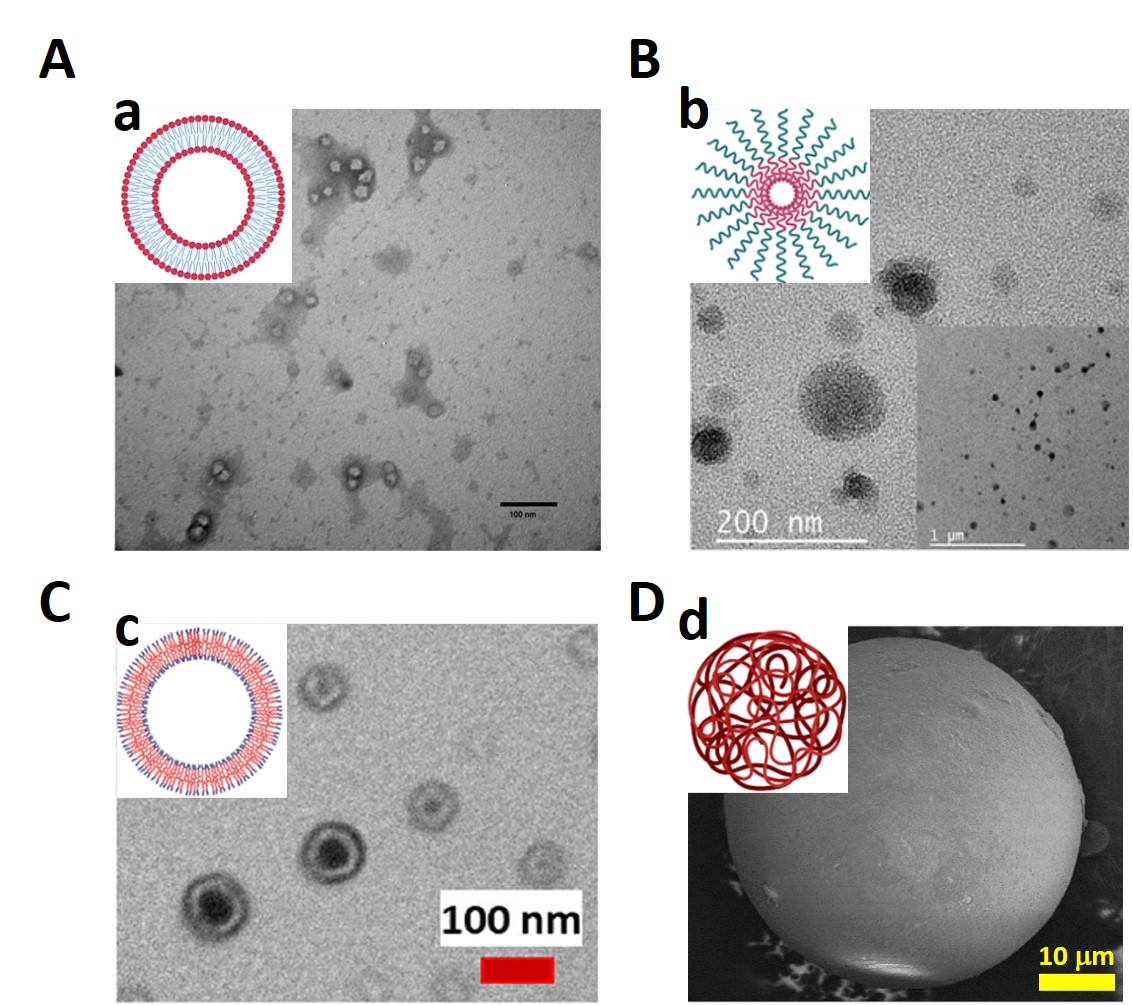
3. Polymeric Micro/Nanomicelles
4. Micro/Nanopolymersomes
5. Polymeric Micro/Nanospheres
This entry is adapted from the peer-reviewed paper 10.3390/polym13223920
References
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760.
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R. Agarose-Based Biomaterials for Advanced Drug Delivery. J. Control. Release 2020, 326, 523–543.
- Bui, T.; Phan, A.; Cole, D.R.; Striolo, A. Transport Mechanism of Guest Methane in Water-Filled Nanopores. J. Phys. Chem. C 2017, 121, 15675–15686.
- Tian, Z.; Yu, Q.; Xie, Y.; Li, F.; Lu, Y.; Dong, X.; Zhao, W.; Qi, J.; Wu, W. Controlling Release of Integral Lipid Nanoparticles Based on Osmotic Pump Technology. Pharm. Res. 2016, 33, 1988–1997.
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of Drug Release from Advanced Drug Formulations Such as Polymeric-Based Drug-Delivery Systems and Lipid Nanoparticles. J. Pharm. Investig. 2017, 47, 287–296.
- Liu, J.; Tan, C.S.Y.; Lan, Y.; Scherman, O.A. Aqueous Polymer Self-Assembly Based on Cucurbit Uril-Mediated Host-Guest Interactions. Macromol. Chem. Phys. 2016, 217, 319–332.
- Vauthier, C.; Ponchel, G. Polymer Nanoparticles for Nanomedicines; Springer: Berlin, Germany, 2017; ISBN 3319414194.
- Saucier-Sawyer, J.K.; Seo, Y.-E.; Gaudin, A.; Quijano, E.; Song, E.; Sawyer, A.J.; Deng, Y.; Huttner, A.; Saltzman, W.M. Distribution of Polymer Nanoparticles by Convection-Enhanced Delivery to Brain Tumors. J. Control. Release 2016, 232, 103–112.
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663.
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298.
- Chapman, R.; Stenzel, M.H. All Wrapped up: Stabilization of Enzymes within Single Enzyme Nanoparticles. J. Am. Chem. Soc. 2019, 141, 2754–2769.
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M. Use of Nanotechnology for the Bioremediation of Contaminants: A Review. Processes 2020, 8, 826.
- Lee, K.M.; Kim, K.H.; Yoon, H.; Kim, H. Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers 2018, 10, 551.
- Fletcher, N.L.; Kempe, K.; Thurecht, K.J. Next-generation Polymeric Nanomedicines for Oncology: Perspectives and Future Directions. Macromol. Rapid Commun. 2020, 41, 2000319.
- Zhang, W.; Hong, C.; Pan, C. Polymerization-induced Self-assembly of Functionalized Block Copolymer Nanoparticles and Their Application in Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800279.
- Wu, S.; Yang, Z.; Fang, S.; Tang, Z.; Liu, F.; Guo, B. Malleable Organic/Inorganic Thermosetting Hybrids Enabled by Exchangeable Silyl Ether Interfaces. J. Mater. Chem. A 2019, 7, 1459–1467.
- Pan, D.; Vargas-Morales, O.; Zern, B.; Anselmo, A.C.; Gupta, V.; Zakrewsky, M.; Mitragotri, S.; Muzykantov, V. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PLoS ONE 2016, 11, e0152074.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403.
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-Based Drug Co-Delivery Systems in Cancer Cells. Mater. Sci. Eng. C 2017, 71, 1327–1341.
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear Imaging of Liposomal Drug Delivery Systems: A Critical Review of Radiolabelling Methods and Applications in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160.
- Johnsen, K.B.; Gudbergsson, J.M.; Duroux, M.; Moos, T.; Andresen, T.L.; Simonsen, J.B. On the Use of Liposome Controls in Studies Investigating the Clinical Potential of Extracellular Vesicle-Based Drug Delivery Systems—A Commentary. J. Control. Release 2018, 269, 10–14.
- El-Hammadi, M.M.; Arias, J.L. An Update on Liposomes in Drug Delivery: A Patent Review (2014–2018). Expert Opin. Ther. Pat. 2019, 29, 891–907.
- Guo, X.; Zheng, H.; Guo, Y.; Wang, Y.; Anderson, G.J.; Ci, Y.; Yu, P.; Geng, L.; Chang, Y.-Z. Nasal Delivery of Nanoliposome-Encapsulated Ferric Ammonium Citrate Can Increase the Iron Content of Rat Brain. J. Nanobiotechnology 2017, 15, 1–13.
- Mejía De Los Ríos, S.P.; Sánchez Toro, A.; Vásquez, V.; Orozco Holguín, J. Functional Nanocarriers for Delivering Itraconazole against Fungal Intracellular Infections. Front. Pharmacol. 2021, 12, 1520.
- Khan, R.U.; Yu, H.; Wang, L.; Zhang, Q.; Xiong, W.; Nazir, A.; Fahad, S.; Chen, X.; Elsharaarani, T. Synthesis of Polyorganophosphazenes and Preparation of Their Polymersomes for Reductive/Acidic Dual-Responsive Anticancer Drugs Release. J. Mater. Sci. 2020, 55, 8264–8284.
- Cerón, A.A.; Nascife, L.; Norte, S.; Costa, S.A.; do Nascimento, J.H.O.; Morisso, F.D.P.; Baruque-Ramos, J.; Oliveira, R.C.; Costa, S.M. Synthesis of Chitosan-Lysozyme Microspheres, Physicochemical Characterization, Enzymatic and Antimicrobial Activity. Int. J. Biol. Macromol. 2021, 185, 572–581.
- Moffitt, M.; Khougaz, K.; Eisenberg, A. Micellization of Ionic Block Copolymers. Acc. Chem. Res. 1996, 29, 95–102.
- Manjappa, A.S.; Kumbhar, P.S.; Patil, A.B.; Disouza, J.I.; Patravale, V.B. Polymeric Mixed Micelles: Improving the Anticancer Efficacy of Single-Copolymer Micelles. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 1–58.
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with Ultralow Critical Micelle Concentration as Carriers for Drug Delivery. Nat. Biomed. Eng. 2018, 2, 318–325.
- Umapathi, R.; Venkatesu, P. Thermo-Responsive Triblock Copolymer Phase Transition Behaviour in Imidazolium-Based Ionic Liquids: Role of the Effect of Alkyl Chain Length of Cations. J. Colloid Interface Sci. 2017, 485, 183–191.
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical Stimuli-Responsive Vesicles in Drug Delivery: Beyond Liposomes and Polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275.
- Oz, U.C.; Kucukturkmen, B.; Ozkose, U.U.; Gulyuz, S.; Bolat, Z.B.; Telci, D.; Sahin, F.; Yilmaz, O.; Bozkir, A. Design of Colloidally Stable and Non-Toxic Petox-Based Polymersomes for Cargo Molecule Encapsulation. ChemNanoMat 2019, 5, 766–775.
- Iatridi, Z.; Angelopoulou, A.; Voulgari, E.; Avgoustakis, K.; Tsitsilianis, C. Star-Graft Quarterpolymer-Based Polymersomes as Nanocarriers for Co-Delivery of Hydrophilic/Hydrophobic Chemotherapeutic Agents. ACS Omega 2018, 3, 11896–11908.
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering Polymersomes for Diagnostics and Therapy. Adv. Healthc. Mater. 2018, 7, 1701276.
- Lei, L.; Bergstrom, D.; Zhang, B.; Zhang, H.; Yin, R.; Song, K.-Y.; Zhang, W. Micro/Nanospheres Generation by Fluid-Fluid Interaction Technology: A Literature Review. Recent Pat. Nanotechnol. 2017, 11, 15–33.
- Abdelkarim, M.; Abd Ellah, N.H.; Elsabahy, M.; Abdelgawad, M.; Abouelmagd, S.A. Microchannel Geometry vs Flow Parameters for Controlling Nanoprecipitation of Polymeric Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125774.
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of Chitosan Microspheres for Efficient Adsorption of Methyl Orange. Chin. J. Chem. Eng. 2018, 26, 657–666.
 Encyclopedia
Encyclopedia
