You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Pulmonary hypertension (PH) is a serious hemodynamic condition, characterized by increased pulmonary vascular resistance (PVR), leading to right heart failure (HF) and death when not properly treated. The prognosis of PH depends on etiology, hemodynamic and biochemical parameters, as well as on response to specific treatment.
- pulmonary hypertension
- chronic thromboembolic pulmonary hypertension
- pulmonary arterial hypertension
1. Introduction
Pulmonary hypertension (PH) is a progressive, heterogenous disease, characterized by increased pulmonary vascular resistance (PVR), subsequently leading to elevated pulmonary arterial pressure (PAP) and increased workload of the right ventricle (RV). The RV adapts to the pathological afterload by increasing wall thickness and contractility. However, the compensatory mechanisms may fail, resulting in right heart failure (HF) and death, if not properly treated.
Consistent with the European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines, there are five groups of PH, according to clinical and pathophysiological criteria. Group 1 contains idiopathic pulmonary arterial hypertension (iPAH), as well as drug-induced PAH and all heritable forms of PAH. Group 2 is PH secondary to left-sided heart failure. PH in group 3 is caused by lung disease and/or hypoxia, and group 4 is chronic thromboembolic pulmonary hypertension (CTEPH). Group 5 consists of PH due to uncertain multifactorial mechanisms. Targeted pharmacological or interventional treatment can be offered to patients diagnosed with PAH and CTEPH, respectively [1,2]. The prognosis of PH varies broadly and depends mostly on etiology of PH, but is also based on hemodynamic and biochemical parameters, which indicate the severity of right ventricular failure, as well as on response to specific treatment. Early recognition of the disease and risk stratification seem to be crucial to identifying patients at high risk and optimizing therapeutic management. Thus, biomarkers may specifically indicate the disease and provide information about the disease stage and treatment response in a relatively easily accessible and noninvasive way. The search for novel emerging biomarkers is still ongoing, resulting in a few potential biomarkers mirroring numerous pathophysiological courses. The main focus is on detecting and quantifying abnormal adaptations and remodeling of the right heart in response to chronic pulmonary circulation impairment. However, in the natural course of PH and right ventricular HF, tissue damage, fibrosis, inflammation, and endothelial dysfunction seem to be also crucial underlying mechanisms, which may be included in noninvasive biomarker assessment (Figure 1). In the present article, we review circulating biomarkers related to different mechanisms underlying the precapillary PH and describe the potential application for them, highlighting their limitations and necessity for further investigation.
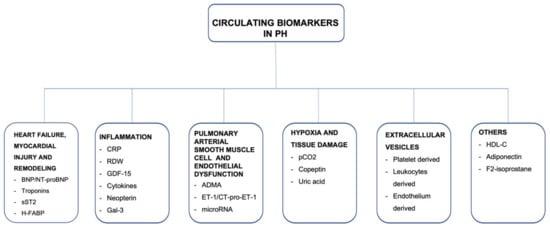
Figure 1. A summary of circulating biomarkers in precapillary pulmonary hypertension.
2. Biomarkers Related to Heart Failure, Myocardial Injury, and Remodeling
In PH, elevated PVR and PAP lead to hemodynamic stress, myocardial strain, and stretching of the heart. Consequently, this condition results in the release of the molecular mediators, indicative for numerous cardiovascular diseases with additional prognostic value. Several markers associated with HF, myocardial injury, and myocardial remodeling, such as natriuretic peptides, cardiac troponins, soluble ST2, and heart-type fatty acid-binding protein, have been investigated in a cohort of patients with precapillary PH.
2.1. Natriuretic Peptides
Brain-type natriuretic peptide (BNP) is produced as an inactive precursor (proBNP), then converted into the active form N-terminal-pro brain-type natriuretic peptide (NT-proBNP) and released from cardiomyocytes. Due to the longer half-life of NT-proBNP compared to BNP, NT-proBNP is preferred in clinical practice as a marker of heart overload and myocardial dysfunction [3,4]. NT-proBNP remains a well-established and widely used biomarker in numerous cardiovascular diseases. It is released in response to ventricular wall stress and myocardial hypoxia or ischemia. NT-proBNP is mostly used in the diagnostic process of patients with acute or chronic HF as well as in predicting prognosis of those patients [5]. In PH patients, serum NT-proBNP levels correlate with right heart dysfunction and provide prognostic information at diagnosis and during follow-up assessment [6,7,8]. However, due to the high variability of NT-proBNP levels and its possible inadequate correlation with hemodynamic parameters and exercise capacity, it should only be interpreted in the clinical context [6]. At present, NT-proBNP is a crucial element of risk stratification in PAH patients and is addressed in both the risk score developed from the REVEAL registry (Registry to Evaluate Early and Long-Term PAH Disease Management) [9,10] and in the risk stratification method proposed by ESC/ERS guidelines [2]. Consistent with REVEAL registry data, a baseline NT-proBNP level of ≤340 ng/L is a strong predictor of improved survival up to 5 years in PAH patients [10]. In slightly different terms, the ESC/ERS guidelines classify NT-proBNP concentrations as low (<5%), intermediate (5–10%), or high (>10%) risk of 1-year mortality, by using specific thresholds of 300 and 1400 ng/L [2]. A significant decrease in NT-proBNP levels among patients with PAH is associated with the response to targeted medical therapy [11,12]. In CTEPH patients, BNP may not only reflect the degree of RV dysfunction and hemodynamic severity of the disease, but also facilitate to assess the effect of pulmonary endarterectomy (PEA) [13], with estimated BNP baseline cut-off values predictive of worse postoperative survival [14]. Furthermore, both balloon pulmonary angioplasty (BPA) as well as pharmacological treatment result in a decrease of NT-proBNP concentration [15,16,17]. In patients treated with BPA, a reduction in NT-proBNP concentration is associated with a significant decrease in mean PAP and PVR, thereby indicating the procedural success of BPA [15]. Table 1 and Table 2 presents changes in BNP and NT-proBNP concentrations before and after BPA treatment in the hitherto published case series.
Table 1. Changes in BNP levels in CTEPH patients before and after BPA treatment in the hitherto published case series.
| Studies | No. of Patients (n) | No. of BPA Sessions (n) | BNP before BPA (pg/mL) | BNP after BPA (pg/mL) | p |
|---|---|---|---|---|---|
| Sugimura et al. [18] | 12 | NR | 335 ± 105 | 16 ± 11 | S |
| Kimura et al. [19] | 66 | 446 | 237.7 ± 475.7 | 45.2 ± 47.6 | S |
| Ogo et al. [20] | 80 | 385 | 227 ± 282 | 48 ± 57 | S |
| Yamasaki et al. [21] | 20 | 2.7 per pt | 66.5 ± 61.3 | 33.8 ± 30.0 | S |
| Aoki et al. [22] | 24 | 113 | 112 (49–199) | 27.5 (14.6–58.4) | S |
| Inami et al. [23] | 103 | 350 | 94 (42–232) | 61 (39–150) | S |
Data are presented as mean ± SD or median and (IQR), S—p < 0.05; BPA—balloon pulmonary angioplasty; BNP—brain natriuretic peptide.
Table 2. Changes in NT-proBNP levels in CTEPH patients before and after BPA treatment in the hitherto published case series.
| Studies | No. of Patients (n) | No. of BPA Sessions (n) | NT-proBNP before BPA (pg/mL) | NT-proBNP after BPA (pg/mL) | p |
|---|---|---|---|---|---|
| Kurzyna et al. [24] | 31 | 117 | 2571 ± 2719 | 634 ± 697 | S |
| Olsson et al. [25] | 66 | 446 | 504 (233–1676) | 242 (109–555) | S |
| Araszkiewicz et al. [26] | 15 | 71 | 1554.8 ± 1541.3 | 537 ± 642.6 | S |
| Darocha et al. [27] | 70 | 377 | 1307 (510–3294) | 206 (83–531) | S |
| Gerges et al. [28] | 45 | 6 (4–10) per pt | 579 (182–1385) | 198 (70–429) | S |
Data are presented as mean ± SD or median and (IQR), S—p < 0.05; BPA—balloon pulmonary angioplasty; NT-proBNP—N-terminal-pro brain-type natriuretic peptide.
2.2. Cardiac Troponins
So far, both cardiac troponin I (cTnI) and T (cTnT) are the principal biomarkers for the detection of myocardial damage and key factors in the diagnosis of acute myocardial infarction [29]. In addition, the development of high-sensitivity assays has made it possible to detect troponin concentrations and their association with morbidity and mortality in many chronic diseases, such as heart failure, coronary artery disease, or chronic kidney disease [30,31,32,33]. Although the underlying mechanisms for troponin release in some conditions remain not completely elucidated, in most cases troponins levels correlate with markers of left heart structural abnormalities and other markers related to left HF. In contrast, the mechanism of troponin release in PH patients seems to be associated with RV pathology, seemingly caused by demand–perfusion mismatch or microcirculation impairment. These theories are supported by the results from several research studies, in which significant correlations between troponins concentration and hemodynamic parameters, including mean PAP (mPAP), mixed venous oxygen saturation (mvSatO2), and RV ejection fraction, were identified [34,35]. Moreover, both cTnT and cTnI concentrations were associated with worse outcomes in mixed cohorts of PH patients [34,36]. Thereby, ESC guidelines indicate that for comprehensive prognostic assessment and risk stratification, troponin levels should be measured at the diagnosis of PAH, then at least once a year or whenever the patient presents with clinical worsening [2]. In CTEPH patients undergoing interventional treatment with BPA, high-sensitivity cTnT concentration decreases stepwise under therapy, signifying a reduction of ongoing myocardial damage due to decreased right ventricular afterload after BPA therapy [37]. Thus, also in the CTEPH patient population, troponins can be a useful marker to monitor the progress of treatment.
2.3. Soluble ST2
Soluble ST2 (sST2) protein is another promising biomarker in PH patients. Protein ST2 belongs to the Toll interleukin 1 receptor superfamily and exists in two isoforms: transmembrane ST2 ligand (ST2L) and soluble ST2 (sST2), which circulates in the blood. The transmembrane form is expressed mainly on inflammatory cells and takes part in strengthening of the immune response of Th2 lymphocytes. However, it is also exposed in cardiomyocytes and endothelium [38]. The ligand for ST2 is interleukin 33 (IL-33), whose expression increases due to mechanical overload and ischemia of cardiomyocytes [38]. The paracrine IL-33/ST2L system plays a protective role, counteracting fibrosis and myocardial hypertrophy. The sST2 protein, which prevents IL-33 binding to the ST2L, is responsible for interrupting this protective action. The balance between both isoforms of ST2 protein ensures the correct biological effect [38,39]. The increase of sST2 concentration in plasma is associated with cardiac remodeling and hemodynamic stress [38,39,40]. Besides natriuretic peptide family and cardiac troponins, sST2 protein may be an additional biomarker for adverse outcomes in cohorts of patients with acute and chronic heart failure [41,42,43]. The sST2 level above 35 ng/mL in patients with HF is associated with higher risk of adverse events, defined as hospitalization or death in one year, in comparison to subjects with sST2 level below this value [44,45,46]. At present, there is increasing evidence of the use of the sST2 protein for risk stratification in patients with RV failure due to PH. In different types of PH, higher sST2 levels are linked to the remodeling of the RV [47]. In a study involving 100 patients diagnosed with PAH or CTEPH, significant correlations between sST2 and cardiac index (CI), mean right atrial pressure (mRAP), PVR, mvSatO2, NT-proBNP concentration, and 6 min walking distance (6MWD) were noticed [48]. These observations are consistent with those from other studies conducted in smaller populations of patients with precapillary PH [49,50]. Moreover, sST2 has been assessed as a marker of therapy response in 57 CTEPH patients, treated with BPA. In detail, the median sST2 concentration decreased to the range of control group after interventional treatment, but it was not related to the individual grade of response to BPA therapy [51]. In another study, sST2 concentration changed significantly in 37 CTEPH patients treated with BPA in the immediate postprocedural period. Interestingly, in patients who experienced complications in the postprocedural period, the baseline sST2 levels were significantly higher in comparison to those without complications. Thereby, sST2 could be beneficial for preoperative risk assessment in these patients. Furthermore, sST2 concentration significantly increased early after BPA procedure, irrespective of complications. In contrast, no analogous changes in NT-proBNP levels were noticed, which may be suggestive of an additional noncardiac source of sST2 in CTEPH patients. Therefore, in PH, sST2 as a complex biomarker may reflect not only the heart condition but also pulmonary vascular system and lung tissue [52]. Table 3 summarizes the main differences between sST2 and NT-proBNP in management of PH.
2.4. Heart-Type Fatty Acid-Binding Protein
Heart-type fatty acid-binding protein (H-FABP) is a low-molecular-weight protein, which is expressed in the cytosol of cardiomyocytes. H-FABP appears to be a marker of injury of cardiomyocytes and is also considered as additional biomarker for early diagnosis of acute coronary syndrome [53]. Of note, Puls et al. described H-FABP as a suitable marker for risk assessment in patients with acute pulmonary embolism [54]. However, there are only limited data about the application of H-FABP in PH patients. Lankeit et al. examined the role of H-FABP in risk stratification in CTEPH patients. The results of the study revealed H-FABP as an independent marker of adverse outcomes, defined as persistent PH after PEA, CTEPH-related death, or lung transplantation [55]. In contrast, Mirna et al. identified H-FABP as an indicator of postcapillary, but not precapillary PH [56]. Although these initial reports appear promising, further studies enrolling a larger population are needed in order to evaluate existing discrepancies.
3. Markers of Inflammation
There is increasing evidence that inflammation processes have great significance in the pathophysiology of PH, being involved in pulmonary arterial remodeling. However, the inflammatory component could also mirror organs distress caused by a certain degree of ischemia and elevated sympathetic drive as a consequence of limited cardiac output. A variety of both anti- and proinflammatory molecules have been investigated as potential biomarkers in cohorts of PH patients.
3.1. C-Reactive Protein
C-reactive protein (CRP), a widely used marker of inflammation, is broadly established as a predictor of numerous cardiovascular diseases, including different types of PH. In PAH, significant correlations between CRP and RAP, 6MWD as well as NYHA class were revealed [57]. Scognamiglio et al. observed that in patients with congenital heart disease-associated PAH (CHD-PAH), CRP concentration was commonly increased and the CRP elevation above 10 mg/mL was associated with around four times greater risk of death [58]. Wynants et al. examined CRP effects on pulmonary vascular cells in CTEPH patients. They revealed that CRP could play a role in chronic obstruction of pulmonary arteries by stimulating endothelial dysfunction, vascular remodeling, and in situ thrombosis [59]. In CTEPH patients, plasma CRP concentrations were related to tissue factor (TF) antigen, suggesting the connection between thrombosis and inflammatory processes in the pathogenesis of CTEPH [60]. Moreover, Quarck et al. observed that CRP levels were elevated among CTEPH patients and significantly decreased 12 months after PEA [57]. However, due to the reported elevated CRP levels in many clinical conditions, including various cardiovascular diseases, its potential use in the diagnosis and monitoring of PH patients remains limited.
3.2. Red Blood Cell Distribution Width
Red blood cell distribution width (RDW) is a laboratory biomarker of heterogeneity, regularly measured in standard blood analyses. Elevated RDW levels are the sign of anisocytosis, which is linked with underlying inflammatory processes [61]. So far it is known that RDW may be a predictor of survival in various cardiovascular diseases, such as coronary artery disease [62], chronic heart failure [63], or acute pulmonary embolism [64]. Moreover, RDW is a prognostic marker of PH of different etiologies, and an association with mortality in a cohort of PH patients was noticed [65,66]. In study involving 77 inoperable CTEPH and PAH patients, the decrease in RDW level after initiation or escalation of specific treatment was linked with good treatment response and improved prognosis [67]. Similar results were previously obtained by Wang et al. in 56 CTEPH patients [68]. However, there is a need for prospective studies to better assess the prognostic value of RDW in cohorts of patients with precapillary PH.
3.3. Growth Differentiation Factor-15
Growth differentiation factor-15 (GDF-15) is a member of the TGFβ superfamily. GDF-15 is exposed in various types of cells in response to tissue damage, ischemia, or shear stress [69,70]. So far, GDF-15 has been indicated as a nonspecific marker of systemic stress in several cardiovascular diseases [71]. In PH, GDF-15 is present in the plexiform lesions in the pulmonary vascular bed and may thus affect both apoptosis and proliferation of endothelial cells [69]. Nickel et al. revealed that in 22 patients with iPAH, GDF-15 levels were associated with hemodynamic parameters such as RAP and pulmonary capillary wedge pressure (PCWP), as well as with biochemical parameters, such as NT-proBNP concentration. However, there were no significant changes in median GDF-15 levels measured prior to beginning of specific therapy and at three- or six-month follow-up [72]. Furthermore, in a study by Meadows et al. in patients with scleroderma and associated PAH, GDF-15 was a marker of reduced survival and correlated with NT-proBNP levels and right ventricular systolic pressure assessed by transthoracic echocardiography [73]. The observations mentioned above brightly propose that GDF-15 could be a prognostic factor in PAH. GDF-15 was also assessed as a marker in therapy response in CTEPH patients treated with BPA. Kriechbaum et al. revealed no significant changes before and after BPA treatment, but there was a correlation between delta change in GDF-15 levels and the change in CI and RAP. In addition, a low concentration of GDF-15 measured at baseline indicated responders to the BPA therapy at the follow-up [51].
3.4. Cytokines
Various cytokines are considered crucial inflammatory mediators in numerous conditions, including PH. In a study conducted by Soon et al., serum levels of several interleukins (IL), such as IL-1, IL-2, IL-4, IL-6, IL-8, Il-10, and IL-12p70, and tumor necrosis factor-α (TNFα) were higher in patients with PAH in comparison to a group of healthy controls. From the ILs mentioned above, IL-6, IL-8, IL-10, and IL-12p70 were prognostic factors of poor survival in iPAH and familial PAH [74]. These data are consistent with results obtained by Selimovic et al., which revealed significantly higher levels of IL-6, transforming growth factor β1 (TGFβ1), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) in PAH patients compared to controls. Moreover, in this study, a significant association between IL-6 and mortality was observed [75]. Similar observations were previously revealed by Langer et al. in a cohort of CTEPH patients [76]. Elevated levels of IL-6, IL-8, and TNFα were observed in CTEPH patients before PEA. Hence, both IL-6 and Il-8 presented a noticeable peak immediately after PEA, whereas TNFα levels significantly decreased within 24 h after the procedure [76]. What is more, in a study conducted by Zabini et al., significant correlations of IL-6 and hemodynamic parameters and exercise capacity were observed [77].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11020383
This entry is offline, you can click here to edit this entry!
