Periodontal and peri-implant regeneration is the technique that aims to restore the damaged tissue around teeth and implants. They are surrounded by a different apparatus, and according to it, the regenerative procedure can differ for both sites. During the last century, several biomaterials and biological mediators were proposed to achieve a complete restoration of the damaged tissues with less invasiveness and a tailored approach.
- tissue engineering
- periodontal regeneration
- biomaterials
- 3D printing
- growth factors
- regeneration
1. Introduction
The improved quality and expectancy of life of the current population leads to an increase in injuries and bone disease in older people who have a diminished capacity to restore and regenerate the damaged tissues [1]. Oral and craniofacial tissue injuries are still a very challenging situation for dentists and oral surgeons. To facilitate dentists in their clinical practice, tissue engineering is in constant evolution, and each year several biomaterials are proposed to achieve better results in periodontal and peri-implant regeneration [2]. The principal target of periodontal and peri-implant tissue engineering is to regenerate the supporting tissue of the teeth or implants. Tooth loss, bone, and soft tissue remodeling are consequences of an inflammatory process or age-related decay [3][4]. Periodontitis has been estimated in about 27% of the global population, and to restore missing tooth implants, therapy is a primary alternative to mobile prosthesis [5][6][7]. This condition leads the clinician to evaluate the predisposition of bone and supporting tissue around the missing tooth site. In several conditions, this required a regenerative approach before or during the implant insertion [8][9]. Peri-implantitis has been estimated in 20% of the population and is defined as an inflammatory process that occurs around implants with soft tissue inflammation and supporting bone loss [10].
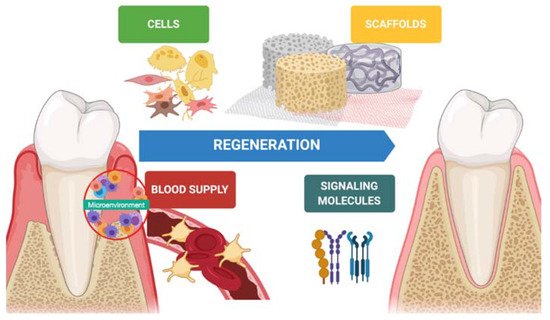
In the last decades, hard tissue regeneration has reached good outcomes regarding newly formed bone, mineralization, and osteoinduction [11]. On the other hand, soft tissue regeneration has also gained interest in preventing advanced forms of periodontitis, peri-implantitis, and mucogingival disorders [12]. Indeed, keratinized tissue, tissue thickness, and supracrestal tissue height around teeth and implants is desirable to achieve better esthetic outcomes and guarantee long-term stability [13][14]. Periodontal regeneration is one of several disciplines that has benefitted from tissue engineering. Biomaterials (scaffolds), molecules (growth factors), and stem cells are keys in the regenerative process, and a synergy between them improves the quality and predictability of the technique ( Figure 1 ).
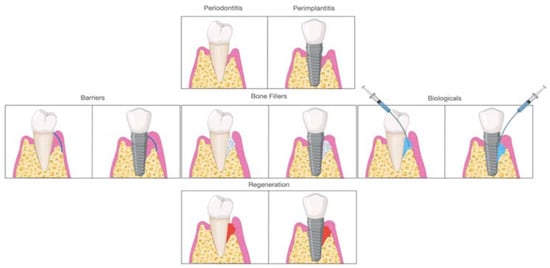
Early on, periodontal regeneration used the concept of guided tissue regeneration (GTR), selecting cell populations to colonize the periodontal wound following surgical exposure [15]. The use of bone substitutes in conjunction with barriers aims to prevent epithelial migration. This allows the periodontal ligament cells (PDL) to populate the protected site, providing positive effects in particular cases [16][17]. Decades of research have expanded from this concept, and different biomaterials are available to clinicians and researchers for alveolar bone regeneration. According to the mechanism of action, biomaterials are classified as barriers, bone fillers, and biologicals. In several situations, such as GTR, treatment components are not used alone but always in combination. Barriers are materials that cover the periodontal defect, protecting them from epithelial downgrowth. Bone fillers are scaffolds or bone grafts that replace the missing portion of the alveolar bone [18]. Biologics are growth factors, cell therapy, or substances that can be directly administrated in the defect ( Figure 2 ).
Efforts have been made over recent years to stimulate bone and soft tissue regeneration around teeth as well as edentulous areas and around implants affected by peri-implantitis [19][20][21].
2. Materials
Grafting materials are commonly used in periodontal tissue engineering to restore the alveolar bone proper, providing adequate regeneration and tooth stability over the years, or the soft and hard tissues around teeth and implants for clinical and esthetic reasons [22]. They include biological and synthetic materials in various shapes and forms, such as granules, particles, gel, 3D scaffolds, injectable substances, polymers, and matrices. According to the type of regeneration and technique, these materials are used alone or in combinations to improve and accentuate the regenerative process. The followed biomaterials were classified and selected according to the scientific evidence extracted from systematic reviews and articles searched on PubMed, Scopus, and Cochrane databases with the following keywords: “Periodontal regeneration” AND/OR “Biomaterials” OR “Peri-implant regeneration” AND “Bone grafts” AND/OR “Biologics” OR “Stem cells”.
Collagen membranes are used not only in periodontal regeneration but also in peri-implant regeneration and, in several cases, regenerative procedures associated with an implantoplasty or heavy decontamination of the implant surfaces ( Figure 3 ).
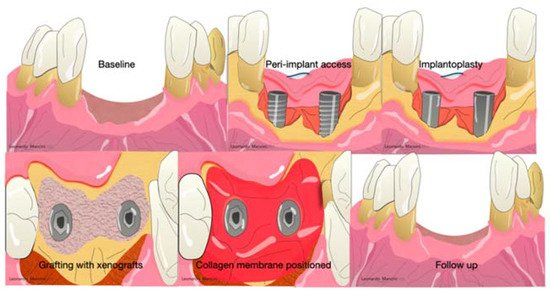
The main advantages of non-resorbable membranes are the high mechanical stability and the cell’s migration inhibition [23]. However, there are some criticisms, such as the second surgical intervention possible exposure, and accentuated inflammation in case of infection [23]. The most widespread and used during the last decade were polytetrafluoroethylene (ePTFE) and titanium-reinforced membranes [24]. PTFE was developed by Gore-Tex (W. L. Gore & Associates, Inc., Newark, Denmark) in 1990. The particularities were the presence of a double layer with different functions; the first layer is porous, and the aim is to promote cell ingrowth. The other side acts as a space provider to inhibit epithelial cell downgrowth. Several randomized clinical trials showed interesting results after three months of healing in periodontal regeneration [25][26][27][28][29][30]. Others reported several complications (exposure, suppuration, pain) probably related to the flap handling and suture collapsing [29]. Nowadays, these barriers are not used due to the introduction of minimally invasive approaches (minimally invasive surgical technique, single flap approach, or modified minimally invasive approach) that can achieve periodontal regeneration without the selection of cells but with the use of growth factors inside the defect associated with a minimal flaps design that maintains the space in favor of blood clot stability [30][31][32][33][34]. Moreover, with these techniques, the handling of a membrane is not easy to obtain.
Biological mediators are considered the last innovation in oral regeneration. It is possible to classify these mediators in stem cells, growth factors, and gene therapy. The most used and widespread are platelet-rich growth factors (PDGF), bone morphogenetic proteins (BMP), and enamel matrix derivatives (EMD).
3. Emerging Technologies
Stem cells are cells of the human body capable of differentiating into any cell of an organism and are self-renewing. They are defined as unspecialized, and, in their evolution, there are various steps of specialization [35]. Research on cell-based approaches is concentrated on the use of mesenchymal stem cells (MSCs), multipotent stem cells with excellent biological proprieties obtainable from nearly all organs and tissues [36].
Periodontal ligament stem cells (PDL-SCs) are used in periodontal ligaments or cementum regeneration. They can be found in alveolar bone and root surfaces, though the PDL-SCs on the alveolar bone show better differentiation abilities. PDL-SCs can differentiate into mesenchymal cell lineages to generate adipocytes, collagen-forming cells, osteoblast-like cells, cementum tissue, and Sharpey’s fibers in vitro [35].
The discovery of periodontal ligament mesenchymal stem cells (PDL-MSCs) into the PDL proposes the important implication of them in the regeneration of the periodontium and its homeostasis. Although the use of PDL-MSCs on bone formation has provided contrasting results, the effect in increasing cementum and PDL formation seems to give good results. This capacity could be supported by the fact that PDL-MSCs express higher levels of various PDL-specific proteins than other MSCs [37]. From a clinical point of view, the use of stem cells is a promising adjuvant in the regenerative procedure. In any case, the limited availability and the requirement of a specialized laboratory render the use limited. In the last three years, a new concept was developed to facilitate the extraction through a mechanical process using simple handling directly in the dental office. Indeed, according to a previous review, this type of extraction seems to be promising in oral regeneration thanks to the combination with scaffolds as collagen membranes or grafts [36]. Studies on stem cells and innovative scaffolds show a potential improvement in term of periodontal regeneration [38][39]. Regarding the use in peri-implant regeneration, preclinical data showed promising results; nevertheless, further clinical studies are needed to validate their effect in peri-implant defects [40].
The introduction of 3D printing in the regenerative field enabled new bioresorbable polymers to be printed and customized for individual cases. The processes are several.
4. Summary and Future Directions
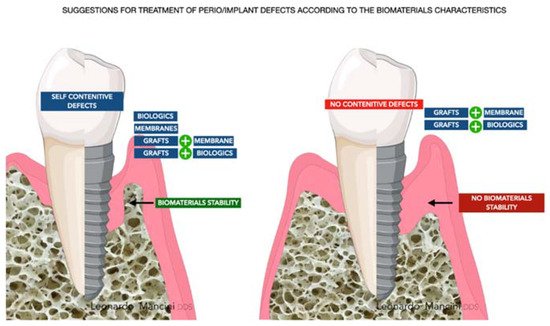
The challenge for each periodontist is to restore all the components of the periodontal compartment (cementum, periodontal ligament, and bone). The regeneration around implants, from the tissue aspect, seems to be easier due to the regeneration of bone only. Nevertheless, it is still a crucial site due to the absence of the anatomical apparatus enriched with vessels, proteins, and growth factors located around teeth. The main elements that need to be considered to have a reliable regeneration are the managing of the occlusal load, mechanical stability of the biomaterial used (grafts better than biologics alone), the reduced FBR due to chemical and thermic treatments that allow the processing of particles, microbiological flora around the defects, dysbiosis control, and, lastly, wound stability. FBR should be discussed carefully due to possible failure related to exposure and infection of the grafts. Exposure is a crucial aspect in daily practice and in many, from an expected regeneration follow-up, has revealed the presence of fibrous encapsulation or graft rejection. The principles, mentioned before, are to be applied for every type of biomaterial and are at the basis of the regenerative process. Moreover, with the introduction of 3D biomaterials and the use of growth factors, signaling is another aspect that needs to be considered to recruit cells and guarantee a proper regenerative response around teeth or implants. However, the main targets are and will be the cost-effective and tailored approaches providing function and esthetics. According to this review for the question “Which is the best biomaterial?”, there is not a specific answer, but the synergetic potential of several biomaterials and the tailored approach lead to a reliable result and predictable regeneration ( Figure 4 ).
This entry is adapted from the peer-reviewed paper 10.3390/ma14123319
References
- Yun, M.H. Changes in regenerative capacity through lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432.
- Sallum, E.A.; Ribeiro, F.V.; Ruiz, K.S.; Sallum, A.W. Experimental and clinical studies on regenerative periodontal therapy. Periodontology 2000 2019, 79, 22–55.
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218.
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontology 2000 2017, 73, 73–83.
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105.
- Romandini, M.; Gioco, G.; Perfetti, G.; Deli, G.; Staderini, E.; Lafori, A. The association between periodontitis and sleep duration. J. Clin. Periodontol. 2017, 44, 490–501.
- Romandini, M.; Shin, H.S.; Romandini, P.; Laforí, A.; Cordaro, M. Hormone-related events and periodontitis in women. J. Clin. Periodontol. 2020, 47, 429–441.
- Schwarz, F.; Giannobile, W.V.; Jung, R.E.; Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 2—Effects of hard tissue augmentation procedures on the maintenance of peri-implant tissues. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 11–13.
- Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Schwarz, F.; Jung, R.; Sanz, M.; Thoma, D. Effects of lateral bone augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 18–31.
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171.
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121.
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Biologics-based regenerative technologies for periodontal soft tissue engineering. J. Periodontal. 2020, 91, 147–154.
- Romandini, M.; Pedrinaci, I.; Lima, C.; Soldini, M.C.; Araoz, A.; Sanz, M. Prevalence and risk/protective indicators of buccal soft tissue dehiscence around dental implants. J. Clin. Periodontol. 2021, 48, 455–463.
- Zucchelli, G.; Tavelli, L.; McGuire, M.K.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J. Periodontal. 2020, 91, 9–16.
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260.
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New attachment formation in the human periodontium by guided tissue regeneration: Case reports. J. Clin. Periodontol. 1986, 13, 604–616.
- Susin, C.; Fiorini, T.; Lee, J.; De Stefano, J.A.; Dickinson, D.P.; Wikesjo, U.M.E. Wound healing following surgical and regenerative periodontal therapy. Periodontology 2000 2015, 68, 83–98.
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2000 2009, 51, 208–219.
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigon, A.; et al. Regeneration of alveolar ridge defects—Consensus report of Group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 277–286.
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 82–91.
- Cosyn, J.; Thoma, D.S.; Hämmerle, C.H.; De Bruyn, H. Esthetic assessments in implant dentistry: Objective and subjective criteria for clinicians and patients. Periodontology 2000 2017, 73, 193–202.
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102.
- Aurer, A.; Jorgic-Srdjak, K. Membranes for periodontal regeneration. Acta Stomatol. Croat. 2005, 39, 107–112.
- Bartee, B.K.; Carr, J. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J. Oral Implantol. 1995, 21, 88–95.
- Marouf, H.A.; El-Guindi, H.M. Efficacy of high-density versus semipermeable PTFE membranes in an elderly experimental model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2000, 89, 164–170.
- Babo, P.S.; Pires, R.L.; Reis, R.L.; Gomes, M.E. Membranes for periodontal tissues regeneration. Ciência Tecnol. Mater. 2014, 26, 108–117.
- Monteiro, A.; Macedo, L.; Macedo, N.-L.; Balducci, I. Polyurethane and PTFE membranes for guided bone regeneration: Histopathological and ultrastructural evaluation. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e401–e406.
- Cortellini, P.; Pini Prato, G.; Tonetti, M.S. Periodontal regeneration of human intrabony defects with titanium reinforced membranes: A controlled clinical trial. J. Periodontol. 1995, 66, 797–803.
- Murphy, K.G. Postoperative healing complications associated with Gore-Tex periodontal material, part I: Incidence and characterization. Int. J. Periodontics Restor. Dent. 1995, 15, 363–375.
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The modified papilla preservation technique: A new surgical approach for interproximal regenerative procedures. J. Periodontol. 1995, 66, 261–266.
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The simplified papilla preservation flap: A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599.
- Cortellini, P.; Tonetti, M.S. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: A novel approach to limit morbidity. J. Clin. Periodontol. 2007, 34, 87–93.
- Trombelli, L.; Farina, R.; Franceschetti, G. Use of the single flap approach in periodontal reconstructive surgery. Dent. Cadmos 2007, 8, 15–25.
- Aimetti, M.; Fratini, A.; Manavella, V.; Giraudi, M.; Citterio, F.; Ferrarotti, F.; Mariani, G.M.; Cairo, F.; Baima, G.; Romano, F. Pocket resolution in regenerative treatment of intrabony defects with papilla preservation techniques: A systematic review and meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2021, 48, 843–858.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Trovato, L.; Naro, F.; D’Aiuto, F.; Moreno, F. Promoting tissue repair by micrograft stem cells delivery. Stem Cells Int. 2020, 2195318.
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of preclinical studies. J. Periodontal Res. 2017, 52, 793–812.
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Oates, T.W.; Chang, X.; Xu, H.H.K. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells 2019, 8, 537.
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® autologous micrografts in oral regeneration: Clinical, histological, and radiographical evaluations. Appl. Sci. 2020, 10, 5084.
- Zheng, R.C.; Park, Y.K.; Cho, J.J.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H. Bone regeneration at dental implant sites with suspended stem cells. J. Dent. Res. 2014, 93, 1005–1013.
