Black fungi are an ecological group of melanized fungi specialized in extremotolerance and assumed to be among the most stress-resistant eukaryotes on Earth. Multi-omics studies have provided significant evidence that they have a peculiar response to stress that differs considerably from that of common mesophilic hyphomycetes. Survival strategies displayed by these organisms have situated them as attractive models for astrobiology and, in general, for studies directed towards the definition of the actual limits for life. Moreover, the ascertained aptitude of black fungi for degradation of hazardous volatile pollutants and for plastic breakdown suggests prospective application of several species.
- astrobiology
- astromycology
- biodegradation
- bioremediation
- black fungi
- black yeasts
- ex-tremophiles
- extremozymes
- plastic degradation
- rock-inhabiting fungi
1. Introduction
Extremophiles occupy environmental niches on the planet that exhibit extremes in physical or chemical conditions. Extreme environments, as considered from the human viewpoint, include myriad niches, habitats, and ecosystems on Earth’s surface and subsurface ranging from deserts to ice sheets [1]. Other examples of extreme environments encompass synthetic habitats that originated as a result of human intervention; these include acid mine waters, sewage and industrial effluents generated by constant discharge of pollutants and toxic waste into the environment [2], and also nuclear reactors, found to harbor microbial life [3].
Extremophiles can be separated into two categories: extremotolerant organisms, which can endure extreme values though growing optimally at “normal” conditions, and extremophilic organisms, which are highly adapted for metabolically and biochemically operating under particular environmental extremes [4].
Extremophiles include members of all three domains of life viz. Bacteria, Archaea, and Eukarya; however, most known extremophiles are microbes. Prokaryotes were for a long time considered the sole colonizers of habitats previously deemed as inhospitable for life. Species belonging to the kingdom Archaea and Bacteria were found to be able to adapt to a variety of extreme settings, including temperature (from 122 °C of hydrothermal vents, i.e., the archaea Methanopyrus kandleri, to frozen sea water at −20 °C, i.e., Psychrobacter cryopegellain), pH (from extreme acid, i.e., the archaea Picrophilusoshimae and Picrophilustorridus can grow at pH 0.06, to extreme basic conditions, i.e., pH 12.8, Halomonas campisalis), high pressure, high metal concentrations, and xerophilic conditions [5]. Some species, such as the bacterium Deinococcus radiodurans and the archaea Halobacterium salinarum, are known as polyextremophiles based on their aptitude to survive multiple stresses, including ionizing radiation [6].
Besides bacteria and archaea, molecular ecology studies additionally brought to light a wide diversity of eukaryotes in different extreme environments, revealing how these organisms are not less adaptable than prokaryotes [7,8]. Protists and the microscopic invertebrate tardigrade are examples of impressive polyextremophiles, however, among eukaryotes, fungi are considered the most versatile and ecologically successful phylogenetic lineage [4]. Whether alone or as lichens, fungi have a great capacity to adapt to a wide range of harsh conditions and are among the most skilled microorganisms to grow in conditions of decreased water availability [9]. Species that thrive in dry ecosystems—where water can be limited due to a low relative humidity, high concentration of solutes or because it is in the form of ice—such as the ascomycete filamentous fungus Xeromyces bisporus, have an absolute requirement for lowered water activity in order to grow [9]; others such as Hortaea wernecki, thrive in hypersaline waters and can grow in nearly saturated salt solution [10]. Similarly, distinctive morpho and physiological features help fungal extremophiles to adapt to extreme cold regions, acidic or deep-sea habitats, and heavily polluted waters [11].
The fascinating lifestyle of extremophiles has fueled much research to understand the mechanisms that enable these organisms to push the limits for life. Advances in molecular biology techniques and the availability of high-throughput DNA sequencing as well as of omics approaches, have contributed to uncovering a hidden abundance of microbial diversity and to revealing the evolution of novel physiologies and biochemistry under extreme conditions. By providing ground-breaking discoveries, the study of extremophiles has stretched the known physical and chemical limits for life and radically changed the understanding toward the conditions under which life can be sustained [12]. Extremophiles have therefore become promising models to further our understanding of the molecular basis of survival and the functional evolution of stress adaptation. Because extremophiles, in particular the hyperthermophiles, are thought to lie close to the nature and behavior of primordial life on the surface of the Earth [13], they are also unique models for astrobiology and exobiology studies exploring the origins of life and the possible existence of life on other planets [14].
Not only are microbial extremophiles of ecological importance, but they also represent a valuable resource for the exploitation of novel biotechnological processes and biomolecules. Proteins, enzymes (extremozymes), and bioactive compounds obtainable from extremophiles are of great interest to biotechnology as they offer advantages over their counterparts from less tolerant organisms in terms of stability and activity (e.g., resistance to proteolysis and recalcitrance to denaturation) [15]. Enzymes produced by thermophiles—e.g., the heat-resistant TaqDNA polymerase from the bacterium Thermus aquaticus—and psychrophiles for instance have received particular attention for their commercial value and multiple industrial uses [16,17]. Myriad applications can be envisioned for enzymes that are stable at extreme values of several physicochemical parameters, including their use for biodegradation and bioremediation purposes in man-made extreme habitats. Hence, it is not hard to imagine that in the future, microbial extremophiles could play a key role in aiding the achievement of the targets of sustainability and bio-based economy [18].
2. Black Fungi: An Exquisite Example of Fungal Extremophiles
Specialized fungi have been isolated in considerable numbers from a variety of extreme environments. Among these, there are several cosmopolitan species belonging to common mold genera e.g., Penicillium, that have been shown to have evolved distinct genotypes and physiological properties in the extreme climate [11]. Additionally, a substantial part of isolates from stressing environments is represented by microcolonial fungi (MCF) and black yeasts.
Grouped under the general name black fungi, these organisms are polyphyletic, being distributed into several orders of the classes of Ascomycota Dothideomycetes (e.g., the order Capnodiales) and Eurotiomycetes (e.g., Chaetothyriales); the first is particularly rich in species thriving at the edges of adaptability, whereas the latter mostly comprises saprophytic and pathogenic fungi [19]. Despite representing a heterogeneous taxonomic cluster, they constitute an ecological group of fungi specialized in extremotolerance, that share a set of traits of which melanin pigmentation is the most prevalent [20]. Habitats where they have been shown to thrive require an admirable ability to cope with multiple sources of stress—e.g., extreme temperatures, salinity and pH, excessive radiation, oligotrophy, and desiccation [21–23]—and are not limited to the natural environment. The isolation of black fungi has also been frequently reported from man-made habitats ranging from sauna facilities to the radioactively contaminated walls of the damaged nuclear reactor in Chernobyl [3,24]. The highest diversity of black fungi has however been observed in rocky environments—e.g., the semi-arid areas of the Mediterranean, the Atacama and the Arizona desert, high Alpine regions, the Arctic, and the dry Antarctic valleys—where microcolonial species, also known as rock inhabiting fungi (RIF), colonize exposed rocks surfaces or live in association with lichens and cyanobacteria in cryptoendolithic communities [25–30]. Conversely, several black yeasts species have a life cycle in association with plant, animal, and human hosts; here, stress-tolerance is therefore essential for pathogenic and opportunistic species to establish infection [31,32].
The extraordinary ability of black fungi to tolerate extreme physicochemical parameters has been widely reported. A number of studies conducted on RIF from the Antarctic deserts, primarily by Onofri and Selbmann et al., highlighted how life on exposed rocks in almost permanently frozen conditions implies tolerance toward a combination of stresses. Such stresses include osmotic and freeze-thawing (−20 °C/+20 °C) stress, low water activity, as well as resistance to desiccation (e.g., precipitation in the McMurdo Dry Valleys is represented exclusively by snow that is quickly sublimated) [33] and to strong solar radiations [34]. Colonization of rocks in hot deserts poses similar challenges: temperature fluctuation can be massive (i.e., between −45 and 60 °C), UV irradiation is high, while nutrients and water availability are generally low [35]. Despite this, RIF are very specialized rock settlers and dominant members of the epi- and endolithic microbial populations [36], so much so that species such as Cryomyces antarcticus (Figure 1E) are assumed to be among the most stress-resistant eukaryotes on Earth [37,38].

Figure 1. Typical colony morphologies of black yeasts and microcolonial fungi on agar plates. Hortaea werneckii (A); Cladophialophora immunda (B); Exophiala dermatitidis (C); Knufia chersonesos (D); Cryomyces antarcticus (E). Photos (A,E) by Christian Voitl; (B) by Barbara Blasi; (C,D) by Donatella Tesei. Scale bars: 1 cm.
Broad amplitude of ecological tolerance can also be found among black yeasts, with several species thriving in a wide range of habitats that include the phyllosphere, solar saltern and glaciers (e.g., Aerobasidium pullulans [11]), and even man-made habitats—e.g., steam bath facilities [39], drainpipes, drinking water, and dishwashers [40–42]which are colonized as artificial equivalents to the natural niches (e.g., Exophiala dermatitidis [43]). Black yeasts belonging to the genera Exophiala and Cladophialophora in particular, have been also isolated from various hydrocarbon polluted environments such as industrial spills, car gasoline tanks and air biofilters. Several of these species are opportunistic human pathogens that can cause neurotrophic infections, and it is currently hypothesized that this dual ecology may result from the ability to use aromatic hydrocarbons, and thus also monoaromatic catecholamine neurotransmitters found in the brain, for their own energy metabolism [44,45].
The investigation of the limits for survival in black fungi by various stress simulation experiments, demonstrated how these organisms are able to withstand physicochemical stress factors even far beyond those in their natural habitats [15]. Lethal temperatures of above 70 °C were detected in black yeasts and survival up to 120 °C was observed in species of the genera Capnobotryella, Coniosporium, Exophiala, Hortaea, Phaeotheca, Sarcinomyces, Taeniolella and when in a completely desiccated state [46]. Ability to grow at pH values down to 0 was reported for Acidomyces acidophilus and for Hortaea acidophila [23]. In the genus Hortaea, the H. werneckii species (Figure 1A) has been identified as one that includes the most halotolerant fungi known to date: despite a growth optimum of 1.0–3.0 M NaCl [22], the yeast can grow in nearly saturated salt solutions, as well as without sodium chloride [11]. Similarly, a remarkable flexibility was observed toward temperature in strains of the genus Elasticomyces, isolated from Antarctic lichens, that unlike all known Antarctic psychrophilic strains (optimum at 15 °C), are capable to grow at 0 as well as at 25 °C [23]. Some species of black yeasts e.g., Exophiala dermatitidis (Figure 1C)and black fungi e.g., Friedmanniomyces endolithicus, were found to display high resistance when exposed to acute ionizing radiations even far beyond the values found naturally in the environment [3,47]. Others have been recognized biodegradation and bioremediation potential [44,48,49]. Further investigations proved the aptitude of black fungi, in particular RIF, to endure simulated Mars and space conditions and to remain viable even after prolonged exposure to outer space [50–54].
3. Life Finds Its Way: Mechanisms of Stress Tolerance in Black Fungi
3.1. Morphophysiological Traits
A number of morphophysiological characteristics underpins black fungi stress tolerance (Figure 2A–E). The switch among different growth forms is a key adaptation that allows these organisms to cope with changing physical-chemical conditions [20,55]. Phenotypic plasticity is observed in many species: according to the circumstances of the habitat, some alternate filamentous and yeast growth form, others transition to completely budding forms (Figure 3) [56]. Additionally, microcolonial growth is typically observed in rock-inhabiting species, where the mycelium becomes compact to form clump-like colonies of thick-walled, heavily melanized cells. Microcolonial development reportedly shelters black fungi from desiccation and heat by optimizing the volume–surface ratio and is adopted as stable character under permanent stress [57,58]. Black fungi generally resort to strategies to minimize efforts at both the morphological and physiological level when exposed to stress, which translates into simple life cycles—e.g., rock inhabiting species are invariably asexual—and minimal morphological differentiation [59]. The absence of sporulation and conidiation observed in several microcolonial species, results in the use of each single vegetative cell as both a survival and a dispersal state and represents a crucial energy-saving mechanism under unfavorable life conditions [15,60].
Another prominent phenotypic feature of all black fungi, identified as a major stress protective compound, is melanization. Melanins are a group of polymeric secondary compounds that can be produced from different precursors and biosynthetic pathways. Those more frequently found in ascomycetes are DHN-melanins (1,8-dihydroxynaphthalene), synthesized from acetyl-CoA (allomelanins), but DOPA-melanins (3,4-dihydroxyphenylalanine), whose precursor is tyrosine, occur as well (eumelanins) [61]. Their accumulation in the cell wall is responsible for the typical green to black color of the mycelium, while their functions include protection against UV- and ionizing-irradiation, extreme pH, and osmotic stress. The radioprotective properties of fungal melanins stem from a combination of a barrier-like physical shield, and chemical shielding by means of their radical scavenging activities [3]. Moreover, melanin electrochemical properties change in response to irradiation [62]. On these premises, it was proposed that enhanced survival and improved fitness of black fungi upon exposure to ionizing radiation may be due to a melanin-dependent mechanism whereby radiation is transduced into other usable forms of energy to support growth [63]. Melanins seem to also increase fungal tolerance toward metals: by binding metal ions, they decrease the ions concentration and thereby allow fungi to also grow in contaminated environments [19]. Moreover, melanins play a major role in pathogenic and opportunistic species: not only by reducing the fungus susceptibility to the host immune system [64] but also by having an essential biomechanical function in facilitating penetration of the host tissues [65]. Melanins’ role in osmo-adaptation appears evident when considering that fungi that tolerate hypersaline conditions are almost exclusively melanized. In this regard, it has been hypothesized that the melanin layer in the cell wall may be crucial to reducing loss of osmoprotective substances (e.g., glycerol) during salt stress, by decreasing cell permeability [66].
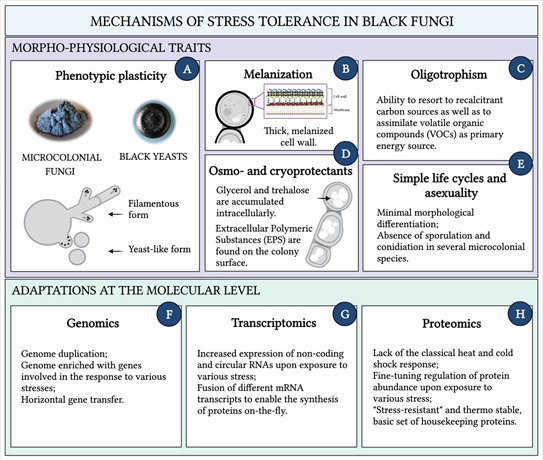
Figure 2. Morphophysiological characteristics and adaptations at the molecular level, that underpin black fungi stress tolerance. The ability to switch between filamentous and yeast-like forms and to grow meristematically (A); Deposition of melanins in the multilayered cell wall. Major constituents of the wall are shown. From bottom to top: the chitin (in red)/glucan matrix, melanin (in black) and the glycoprotein-rich outer layer. The composition of the cell wall varies between species as well as the location of the melanin layer. (B); The aptitude to thrive in environments in which factors required for growth are scarce (C); The synthesis of life-saving compounds, which are accumulated both intra- and extracellularly. Large intracellular globules of trehalose and glycerol can fill the whole cytoplasm; (D); Energy-saving strategies to minimize efforts at both the morphological and physiological level under unfavorable life conditions (E); Molecular mechanisms for stress-resilience at the genomic, transcriptomic, and proteomics level (F–H). Created with BioRender.com.
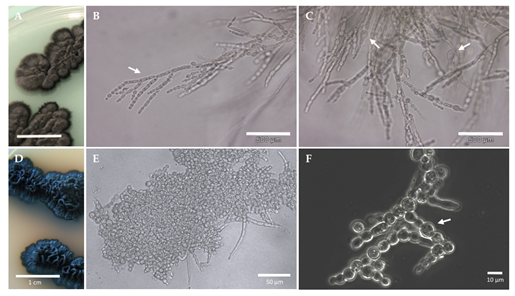
Figure 3. Growth forms observed in black yeasts and black fungi. The black yeast Exophiala dermatitidis (A–C); The microcolonial rock-inhabiting fungus Knufia chersonesos (D–F). Olive-brown colonies growing on 2% malt extract agar (MEA) (A); Nuclei and hyphal growth are visible in (B), Isodiametric expansion (swelling) in hyphal cells is shown in (C). Black, cerebriform colonies growing on 2% MEA (D); Clusters of cells occasionally branching into torulose hyphae (E); Swelling observed in torulose hyphae (F).
Production of osmo- and cryoprotectants is a trait spread across the black fungi group. Although glycerol is generally accumulated intracellularly by halotolerant species to prevent loss of water in highly concentrated salt solutions, a different compatible solute, the disaccharide trehalose, is abundantly produced during both freezing and desiccation [59]. Trehalose acts as stabilizer of enzymes and phospholipidic bilayers, allowing black fungi to survive even complete dehydration [50]. In addition, extracellular polymeric substances (EPS) are abundantly produced by black fungi. Often in the form of a coat on the colony surface, EPS shelter the cells from damage due to repeated freeze-thaw cycles and moreover, their hygroscopic nature also provides protection from dry conditions [67]. The synthesis of these life-saving compounds generally implies high metabolic costs, costs that can be even higher for species populating highly oligotrophic environments. As a result, these fungi typically show a very slow growth rate and may opt for survival strategies that include the slow-down of the metabolic activity until favorable conditions are re-established [20]. At the same time, it is the combination of their reduced growth rates and their marked oligotrophism that enable black fungi to thrive in environments in which factors required for growth are scarce [68]. This is especially evident in RIF and in some opportunistic black yeasts from the order Chaetothyriales, whose survival depends on the ability to resort to recalcitrant carbon sources spurned by other microorganisms [19]. On rocks, fungi can mostly rely on inorganic nutrients and might be occasionally confronted with sudden availability of organic nutrients synthesized by primary producers, or provided in the form of dust particles, animal droppings or fossil organic biomass, etc. [29,69]. Additionally, black fungi are reportedly able to also assimilate volatile organic compounds (VOCs) as a primary energy source, which can explain the isolation of several species from polluted and hydrocarbon-rich environments [70,71]. As mentioned above, a possible connection has been highlighted between the metabolism of aromatic hydrocarbons in the environment and neurotropism displayed by pathogenic black yeasts, where hydrocarbon assimilation would represent a virulence factor [72]. Altogether, the extraordinary nutritional plasticity observed in black fungi not only represents a successful survival strategy but also confers these organisms’ biotechnological significance for bioremediation and biodegradation purposes [44,45,48].
3.2. Adaptations at the Molecular Level
Knowledge of morpho- and physiological traits characterizing black fungi has provided a general understanding of these organisms’ polyextremotolerance. However, more recently, the application of genomics, proteomics, and transcriptomics approaches to black fungi research—despite being hampered by the arduous isolation of biomolecules due to the thick and melanized cell walls—has allowed for a better insight into the molecular mechanisms for stress-resilience (Figure 2F–H).
To date, genome sequencing could be performed on only a few of the known species. Interestingly, genome analyses of the Antarctic RIF Cryomyces antarcticus (genome size: 24 Mbp), did not uncover significant deviations from comparative species and mesophilic hyphomycetes. Signatures commonly observed in microbes acclimatized to distinct lifestyles or ecological niches, such as genome duplication or shrinkage, were not detected. The GC content of the DNA, which is generally assumed to reflect the temperature the organisms are exposed to in their natural habitat, does not match the amounts expected in psychrophilic strains; 32 mating genes and 34 mating proteins were identified with protein homologs in the classes Dothideomycetes, Eurotiomycetes, and Sordariomycetes, although sexual reproduction was never observed in this fungus. Even the genes responsible for the biosynthesis of melanin (i.e., laccase and polyketide synthase PKS), “fungal armor” especially in the extremes, are present in a lower number than that detected in comparative species [73]. Although melanin production is essential for the fungus, it was speculated that conservation of the involved genes and thus a lower rate of genetic diversification, could represent an advantage in the natural habitat [74].
Genome sequencing and functional analysis of the Antarctic endemic endolithic RIF Friedmanniomyces endolithicus instead revealed genomic patterns that could be attributed to the fungus stress tolerance [75]. Firstly, the genome size was found to be much larger (i.e., 46.75 Mbp) than that of most other black fungi hitherto sequenced, which is suggestive of evolutionary advantages due to a large-scale genome duplication, to adapt and survive to the prohibitive cold desert conditions. Additionally, the genome appeared enriched with genes involved in response to oxidative stress, UV irradiation, DNA damage, and salt stress; in particular, genomic traits associated to meristematic growth and cold adaptation were found to be unique to this species. Another notable trait, lack of pectinases, suggests that the organisms may have lost the ability to obtain nutrients from plant material in favor of an oligotrophic lifestyle.
Genome duplication was best seen in the halotolerant black yeast Hortaea werneckii, which is thought to have undergone a form of whole genome duplication (WGD) or hybridization between haploid and diploid strains [76]. Indeed, H. werneckii reference genome (EXF-2000) was found to be diploid and consisting of two sub-genomes with a high level of heterozygosity, which is in contrast with what was observed in the majority of fungi, which are instead haploid [74]. The most striking features of the genome (51.6 Mbp) were a large genetic redundancy and several duplications of genes with crucial roles in osmotically stressful conditions, such as those encoding for metal cation transporters [77].
Another example of DNA content reflecting the ecology of a species is the genome of a strain of Cladophialophora immunda sampled from environmental polluted sources (Figure 1B). This species is an opportunistic human pathogen, which is also known for its aptitude for degradation of aromatic compounds such as toluene [78]. Genome analysis resulted in the identification of five clusters of genes involved in toluene degradation. A total of eight genes involved in the toluene-degrading pathway were found to be shared by the black yeast and bacteria of the genus Pseudomonas spp. and four genes were suggested to be horizontally transferred from bacteria [44].
Information obtained on black fungi genomes has been complemented by proteomics data on gene products and their response to a variety of environmental influences. Proteomic analyses performed on a few rock-inhabiting fungi and black yeasts showed that a lack of the classical heat and cold shock response (HSR and CSR, respectively) and in some cases even decreased levels of common stress proteins, representing a key component of black fungi’s response to a variety of suboptimal conditions of growth. Most often, this goes hand in hand with a general down-regulation of the metabolism: a reduction in the total number of expressed proteins represented, for instance, the typical reaction of the Antarctic RIF F. endolithicus and C. antarcticus to temperatures above the growth optimum and desiccation [79,80]. Temperature produced a similar effect in rock fungi from the Mediterranean area e.g., Knufia perforans and Exophiala jeanselmei, although their response to dehydration involved the expression of a few low molecular weight proteins, probably small heat shock proteins (sHSPs) [80]. Similar observations were made in Exophiala dermatitidis, opportunistic black yeast with a broad geographical distribution that ranges from the tropical rain forest to Apennine glaciers (Figures 1C and 3A–C) [81]. Here, 2D- Difference Gel Electrophoresis (DIGE) LC/MS-MS showed no typical stress-response upon supra- and sub-optimal temperature and no protein differential expression following short-term (i.e., 1 h) exposure to temperature stress. A general reduction of the metabolic activity, as well as rearrangements at the cell wall and cell membrane levels, were instead detected after long-term (1 week) exposure to cold [74,82]. These findings are in line with subsequent reports on E. dermatitidis and other black fungi species, e.g., Knufia chersonesos (Figures 1D and 3D–F), which demonstrated how protein intensities do not change substantially in response to a variety of stresses, even well beyond those these organisms normally have to face in their natural environment, including gamma radiation [83], simulated microgravity and Mars-like conditions [51,52], and plastic polymers as sole carbon source [48].
Although the absence of an HSR in Antarctic endemic strains could be attributed to the fact that such a response was not developed during evolution [84], a downregulation of the metabolism and a fine-tuning regulation of proteins abundance represent energy-saving strategies that are generally beneficial in oligotrophic, extreme environments. If protein production has a high energetic demand and can have a great impact on fitness when internal or external stresses are present, the impairment of molecular chaperones, however, can seriously compromise the maintenance of cellular proteostasis [85]. Hence, it can be hypothesized that without the stress-induced upregulation of chaperones and other stress proteins, cell functionality and stability may depend on a basic set of housekeeping proteins capable of remaining functional upon stress exposure [82]. The fact that, in different species of black fungi, few proteins are generally observed changing in their relative amounts in response to given stressors, and moreover, that changes in protein levels are often subtle, suggests that the factors that are responsible for stress resistance are constitutively present [83].
Additional cellular strategies with potential roles in black fungi polyextremotolerance were brought to light by transcriptomics analyses and may involve the modulation of non-coding and circular RNAs (circRNAs), and fusion transcripts. An increase in the expression of non-coding RNAs such as small nucleolar RNAs (snoRNAs) was detected in the transcriptome of the black yeasts C. immunda upon exposure to toluene. Despite the strain being capable of toluene degradation, up-regulation of snoRNAs has been interpreted as a response toward the destabilizing effect of toluene on the maturation process of rRNAs [44]. In an analysis of E. dermatitidis transcriptome, circRNAs and fusion transcripts were found to exhibit temperature-dependent regulation [86]. Though the general functions of circRNAs have not been well uncovered at present, their proven stability to denaturation and to enzymatic degradation may be a crucial component of stress resistance as well as of virulence in black fungi [87]. The aptitude to fuse distinct mRNAs to create the necessary proteins on-the-fly could serve similar purposes.
Besides modulation of basal ncRNAs, a general downregulation of metabolic processes in response to stress has been indicated by transcriptomic analyses in black fungi, which is in line with what was observed at the proteome level. Although often explained by the organisms entering an energy-saving state, Malo et al. [88] observed a correlation between the downregulation of metabolic transcripts and black fungi adaptation to protracted exposure to stress, i.e., ionizing radiations. Their study of a radiation-adapted lab strain of E. dermatitidis showed that downregulation plays a role in reducing the internal ROS load, thereby positioning the yeast to better respond to an external load of ROS.
4. From Stress Adaptation to Biotechnological Significance
4.1. Astrobiology Studies
Survival strategies displayed by black fungi have situated them as attractive model organisms for studies aiming to investigate stress biology and adaptation. Rock-associated species, in particular, are models of interest for the study of the biology and evolution of the rock-inhabiting lifestyle [68]. Rock represents a harsh habitat and a quite ancient niche for life, believed to reflect early Earth conditions and considered to be a model for extraterrestrial life [20]. Hence, as extremophiles, black fungi also serve as model organisms for exobiology and in general, for studies directed towards the definition of the actual limits for life.
Questions about the origin and evolution of life and the possibilities for life beyond planet Earth are the focus of Astrobiology: a multidisciplinary scientific field, which also has practical applications in considering how biological organisms may travel to space in view of space exploration [89]. Astrobiological investigations have a significant impact on how space agencies design missions as well as implications for planetary protection policies, aiming to prevent the introduction of Earth-originated life that may mislead future life detection missions [90]. One of the subjects of astrobiology includes extremophiles: they provide an experimental approach and are also able to act as analogues to potential extraterrestrial life. This seems reasonable, as extremophiles show that the capabilities and ecological range of life’s possibilities are greater than what was assumed before their existence became known [91]. In this frame, rock-inhabiting fungi and especially those living in the closest terrestrial analog for Mars or icy moons, i.e., Antarctica, may possibly mimic a putative life form that might be present elsewhere in the Solar System and beyond [92].
To date, a number of studies have focused on testing black fungi survival to space conditions through simulations in ground-based facilities or in space missions [52]. The first space experiment to include Antarctic RIF (Cryomyces antarcticus and C. minteri) was carried out by Onofri et al. [50] and had the objective of testing the ability of selected organisms to survive long-term space travel [50]. Survivability of C. antarcticus in outer space was shown via colony counts following a 1.5-year-long exposure onboard the EXPOSE-E facility of the Columbus module of the International Space Station (ISS). The viability of this strain, the most resistant one of the two, was around 12.5% as measured by cultural tests. This is remarkable especially considering that space radiation—including ionizing radiations and UV irradiation [93]—is a main source of injury during space journeys outside the ISS [93]. The same research group had previously demonstrated that C. minteri was able to survive simulated Martian atmosphere and pressure, temperature fluctuations between −20 and 20 °C, ultraviolet radiation, and vacuum [37]. A more recent experiment involving C. antarcticus was performed in the frame of the project BIOMEX (BIOlogy and Mars EXperiment), with the aim to investigate microbial survivability as well as DNA and ultra-structural damage [60]. Dried fungal colonies grown on Lunar and Martian regolith analogues were tested for their resistance to strong UV irradiation coupled with space vacuum and temperature cycles, in ground-based simulation experiments. Samples validated for the space mission were subsequently launched to the ISS in view of a 1.5- years exposure to outer space conditions on the EXPOSE-R2 payload. Remarkably, cultural and molecular tests revealed only slight ultra-structural and molecular damage and stated the high stability of DNA within melanized cells [60]. Tests carried out upon sample return to Earth confirmed the viability of C. antarcticus colonies following exposure to space and Mars-like conditions, the latter simulated on the ISS [54]. Survivability ranged from 80% in fully irradiated samples to 40% in samples exposed to Mars atmosphere. Microscopy observations revealed ultrastructural damage, however all the treated samples recovered metabolic activity up to 70%.
In other investigations, the resistance to acute ionizing radiations (x-rays, gamma rays, and heavy ions) was demonstrated in RIF. Although living organisms are not exposed to acute ionizing radiation under natural conditions, tests on C. antarcticus and the cryptoendolithic Antarctic RIF Friedmanniomyces endolithicus revealed that high radiation resistance characterizes both fungi. Irradiation survival (i.e., 12%, by colony forming unit, CFUs count) was shown in C. antarcticus to doses of space-relevant gamma rays up to 55.81 kGy—roughly 10000-fold higher than the lethal dose for humans [94]—together with high DNA stability [53]. Such high resistance, which resulted in the detection of DNA even in non-vital cells, provides support for the use of DNA as a possible biosignature in exploration campaigns. High resistance of the fungus dried colonies was also shown towards accelerated helium ions at doses up to 1.000 Gy, where survival was still 70% and 75% of metabolic activity was maintained. Cell damages were detected at the 1.000 Gy treatment, however good membrane integrity among the cells was generally reported through TEM observation [95].
In F. endolithicus, exposure to 100 Gy gamma rays’ radiation resulted in the death of approximately 40% of cells. However, irradiation with doses up to 400 Gy, did not lead to a statistical difference in cell mortality, suggesting that the fungus may perform inducible damage repair or that F. endolithicus cell population may have heterogeneous radiosensitivity. Radiation exposure exceeding 260 mGy per year in environments has not been found naturally [96]. Thus, the authors speculated that the radiation resistance is a consequence of the fungus adaptation to prolonged desiccation, which, similarly to irradiation, reportedly causes DNA double-strand breaks [97]. Furthermore, they indicated that the high radiation resistance observed in both C. antarcticus and F. endolithicus is also largely related to the presence of melanin in the cell wall [53].
Additional knowledge about the impact of extraterrestrial conditions on the black fungi physiology has been provided by a few systems biology studies investigating changes at the proteome level. A comparative study of 2D protein patterns under simulated Mars-like conditions in ground-based experiments proved C. antarcticus, the mesophilic RIF Knufia perforans, and the rock-colonizing black yeast Exophiala jeanselmei to be metabolically active [51]. Although a significant drop in protein number was detected at first (within 24 h from the beginning of the treatment), on day 4 the protein spot number started to increase again and by day 7, the protein patterns resembled those of the colonies cultured under optimal conditions. Moreover, no additional proteins were expressed under Mars simulation, which could be interpreted as stress-induced HSPs. The authors therefore suggested that along with the already known morpho-physical features of black fungi playing a role in stress protection, survival to Mars-like conditions could also be attributable to a basic set of stress-resistant proteins.
No evidence of significant activation of stress components or starvation was also revealed by the proteome and secretome characterization (Tandem Mass Tag-based quantitative shotgun proteomics) of the RIF Knufia chersonesos exposed to low-shear simulated microgravity (LSSMG) [52]. Microgravity, a condition in which the gravity level is almost zero but not neutralized, is an important factor known to influence microbial gene expression, cell morphology, physiology, and metabolism in space environments [98]. Here, morphological alterations were not detected by microscopy studies following exposure to LSSMG. A comparative analysis of the fungus and its non-melanized mutant (spontaneously originated under laboratory conditions), showed that the mutant mostly engaged in protein downregulation under LSSMG. By contrast, the basic energy metabolism was upregulated in the wild type strain and only subtle rearrangements in the protein repertoire were observed. In line with studies reporting enhanced melanin synthesis as a feature of fungi living on space stations [62,99], the enzyme scytalone dehydratase, involved in the synthesis of DNH melanin, was found to be upregulated in the wild type and downregulated in the mutant. This study therefore indicates the ability of black fungi to cope with LSSMG conditions and suggests that cell wall melanization may ultimately influence the metabolic response to microgravity.
Together, the studies reported above have revealed the capacity of rock-inhabiting black fungi to endure space conditions. Additionally, they showed the bewildering ability of C. antarcticus in particular to survive extraterrestrial conditions over long time scales of applied space exposure, to preserve DNA integrity, and to cope with extraterrestrial regolith analogs, which is of astrobiological significance in the frame of the concept of life transfer between planets (Lithopanspermia theory [100]), Mars habitability, planetary protection, and the search for biosignatures.
4.2. Black Fungi as Biotechnologically Relevant Microorganisms
Although only having emerged as successful technologies for black fungi research in the last decade, multi-omics approaches have greatly contributed to unearthing molecular mechanisms that enable these organisms to push the limits for life. At the same time, the results of omics analyses suggest that black fungi represent a treasure-trove of novel proteins, enzymes, and bioactive compounds that may perform exclusive tasks both in nature and at an industrial level.
The most striking example of this is the black yeast Aureobasidium pullulans. This species has been involved in biotechnological processes for a long time, being harnessed as producers of pullulan (poly-α-1,6-maltotriose), a biodegradable extracellular homopolysaccharide principally used as packaging and coating material, and as food ingredient [101]. A. pullulans is also known to produce the polysaccharide b-glucan, identified as an effective substance to improve animal health conditions, and other metabolites that are used as medical supplements or additives in food. Furthermore, many commercially important enzymes are produced by different strains of A. pullulans: their broad temperature (from 25 to 80 °C) and pH optimum (from 2 to 9) make them suitable for several applications in the food industry [102].
Black fungi have also been investigated in relation to their aptitude for bioremediation and biodegradation. Their role as degraders of hazardous volatile pollutants was initially hypothesized based on their frequent isolation from hydrocarbon-polluted environments. Moreover, experiments of biofiltration of air contaminated with styrene or toluene have led to the enrichment of a number of styrene- and toluene-utilizing species of black yeasts [103]. These findings prompted the development of air biofilters based on using fungi as biocatalysts for the purification of hydrocarbon-polluted air [45]. As a result, an increasing number of fungal strains growing on volatile aromatic hydrocarbons have been isolated, most of which belong to the genera Exophiala and Cladophialophora of the order Chaetothyriales, and their assimilation of toxic aromatics as sole carbon and energy sources has been demonstrated [45,104,105]. Several species commonly found in biofilters are also known as agents of opportunistic infections ranging from localized cutaneous and subcutaneous lesions (e.g., Cladophialophora carrionii and Exophiala spinifera) to neurotrophic infections (e.g., Cladophialophora bantiana and Exophiala dermatitidis) [106]. This pattern of opportunism and assimilation of alkylbenzenes has been observed also in Exophiala xenobiotica, Exophiala lecaniicorni, Exophiala oligosperma, Exophiala mesophila and Phialophora sessilis, among others [45,107]. E. oligosperma, a pathogen associated with phaeohyphomycosis in immunocompromised human hosts [108], appears to be among the dominant species isolated from biofilters treating volatile alkylbenzenes [109]. Similarly, toluene assimilation was demonstrated for a clinical isolate of E. mesophila from a patient with chronic sinusitis [49]. Specifically, it was shown that the strain is able to completely degrade toluene into CO2, with 65% of the C-toluene being recovered as C-CO2. Very similar results (around 65% of the C-toluene recovered as C-CO2) were obtained in the same study for a strain of Cladiophialophora immunda isolated from a polluted soil sample. Genes involved in toluene degradation and stress response mechanisms, which allow the survival to toluene exposure, were additionally identified via the genomic and transcriptomic analysis of the black yeast grown in presence of toluene as sole carbon source (see paragraph 3.2 in this contribution) [44]. In other investigations, removal of volatile organic compounds (VOCs) from indoor air by black yeasts was demonstrated [110]. VOCs are emitted by construction materials and appliances as well as by cleaning products, etc.; their accumulation has been linked to the onset of the sick building syndrome, defined by the World Health Organization as a medical condition that affects people in buildings and whose symptoms are illness or feeling unwell. In the study from Prenafeta et al. (2019) [110], the successful removal of most of the 71 VOCs identified in the indoor air—including xenobiotic and biogenic compounds—was shown in the presence of biomass from Cladophialophora psammophila and Neohortaea acidophila, specialized black yeasts from polluted soils and lignite, respectively. The overall VOCs removal efficiencies were higher than 96% in both tested species.
Several studies therefore suggest that black fungi may represent ideal agents for the bioremediation of polluted habitats, and for the treatment of indoor air or of contaminated gas streams in biofilters. However, further research is still needed to elucidate the ecophysiology of opportunistic and pathogenic species and to determine to what extent hydrocarbon metabolism and virulence may be coincident, in order to guarantee the biosafety of biotechnological applications. The environmental isolation of species of black fungi with potential of toluene assimilation (order Pleosporales, Cladosporiales, and Xylariales) and that are less hazardous than those from the Chaetothyriales is on the other hand very promising for the discovery of novel and safer candidates for bioremediation [111].
Another aspect of biodegradation just recently started to be investigated in black fungi is the breakdown of plastic material. Based on their oligotrophic lifestyle and their ability to degrade simple and polycyclic aromatic compounds, black fungi, and RIF in particular, are potential sources of enzymes with hydrolytic ability towards various types of synthetic polymers. A degradation study carried out using the RIF Knufia chersonesos as model organism [48] revealed that the fungus was able to achieve complete hydrolysis of the synthetic copolyester polybutylene adipate terephthalate (PBAT), commonly used as food packaging and lamination material [112]. Higher levels of hydrolysis products were recorded at minimal medium condition, where the polyester represented the sole carbon source. Analyses of the secretome indicated that several esterolytic and lipolytic enzymes were modulated upon exposure of the fungus to plastic. Out of 37, seven enzymes were detected as upregulated or induced by PBAT in minimal medium cultures. Protein functional analysis and structure prediction proved similarity of some of these enzymes with microbial polyesterases of known biotechnological application—e.g., MHETase from Ideonella sakaiensis, CalA from Candida antarctica, and feruloyl esterase b from Aspergillus oryzae—which is suggestive of their potential role in PBAT degradation. By revealing the aptitude of K. chersonesos for PBAT degradation, these results endorse their biotechnological application in the field of polymer processing, recycling, and degradation [48].
Besides K. chersonesos, other species of black fungi have been indicated as potential degraders of plastic material as well as of plasticizers, commonly organic acid esters embedded in plastics to provide strength and flexibility [113]. A metagenomic analysis identified 10 species, including Baudoinia panamericana, Cyphellophora europaea, E. oligosperma and Rhinocladiella mackenziei, within the microbiome associated with biofouled plastic fabric materials for military and civilian application, exposed to harsh tropical environment [114]. Protein functional annotation revealed hydrolases (esterases and lipases), together with the proteins involved in hydrocarbon degradation (i.e., cytochrome P450 and aromatic ring-opening dioxygenases) and efflux pumps potentially associated with hydrocarbon resistance [115]. The results of this study therefore suggest that the identified species have potential for the degradation of plasticizers as well as of hydrocarbons. Previously, degradation of plasticized PVC (i.e., polyvinyl chloride) was shown in A. pullulans, where the presence of important genes for the degradation of plastics and aromatic compounds was also demonstrated [116,117]. The results of these studies are very promising, however, to date, research on plastic degradation in black fungi is still in its infancy and more studies will be required to screen other species as well as to characterize the proteins of interest.
Additional bioremediation skills of black fungi, which are yet to be fully investigated, are related to melanin. Melanin is known to have properties that facilitate the absorption of heavy metals, making it a good candidate for addressing heavy metal pollution in waterways and the environment [118]. Additionally, based on their resistance against and affinity for radiation—e.g., radiotropism shown by species isolated from the Chernobyl Nuclear Power Plan [119,120]—black fungi may also potentially play a role in the absorption of nuclear pollution, as already observed in other melanized species [121].
5. Conclusions
Due to their localization in extreme niches and remote areas, many black fungi species were not discovered until recent decades. Despite typical traits, such as the slow growth rate of some species and the melanization of the cell wall, having hampered isolation and cultivation of these organisms as well as the extraction of their biomolecules, significant progress has been made in the elucidation of their mechanisms of adaptation to stress, mostly due to the rapid advancement in sequencing technologies and the application of omics approaches. Their biology has widened our views on the diversity of terrestrial life, showing examples of eukaryotes with bewildering capacity to adapt to extreme conditions both on Earth and in space. Whether by revealing their vast bioresource potential or by providing an experimental approach to disciplines such as astrobiology and exobiology, black fungi have rapidly gained prominence as models of great value for both fundamental and applied research. Space experiments in rock-inhabiting species have stretched our concept of the limits for life, providing new insights into the possibility of transfer of life among planets, habitability of extraterrestrial environments, and planetary protection. Moreover, the aptitude of black fungi for removal of contaminants and for plastic breakdown was ascertained and prospective application of several species has been proposed. The growing interest in black fungi research therefore reflects, to some degree, the increasing awareness of climate change and the urge to achieve the target of sustainability through strategies that include recycling and bioremediation, as well as the biotechnological application of enzymes and compounds from natural sources. Continuing to shed light on the potentialities of black fungi may therefore have out-of-this-world implications.
