Inspired by nature, significant research efforts have been made to discover the diverse range of biomaterials for various biomedical applications such as drug development, disease diagnosis, biomedical testing, therapy, etc. Polymers as bioinspired materials with extreme wettable properties, such as superhydrophilic and superhydrophobic surfaces, have received considerable interest in the past due to their multiple applications in anti-fogging, anti-icing, self-cleaning, oil–water separation, biosensing, and effective transportation of water. Apart from the numerous technological applications for extreme wetting and self-cleaning products, recently, super-wettable surfaces based on polymeric materials have also emerged as excellent candidates in studying biological processes.
- nature
- polymeric materials
- super-wettable surfaces
- bioinspired material
- biomedicine
1. Extreme Wetting Surfaces Inspired by Nature and Their Applications in Biomedicine
1.1. Cell Configuration for Studying Cellular Interactions
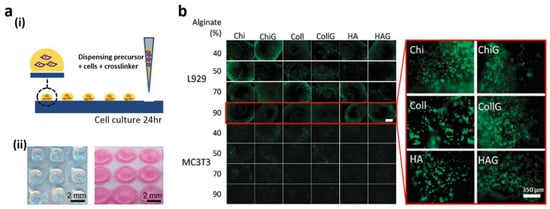
1.2. Functional 3D Cell Spheroids
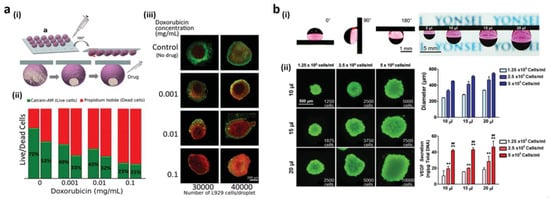
1.3. Biomedical Devices
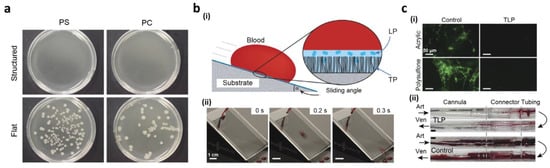
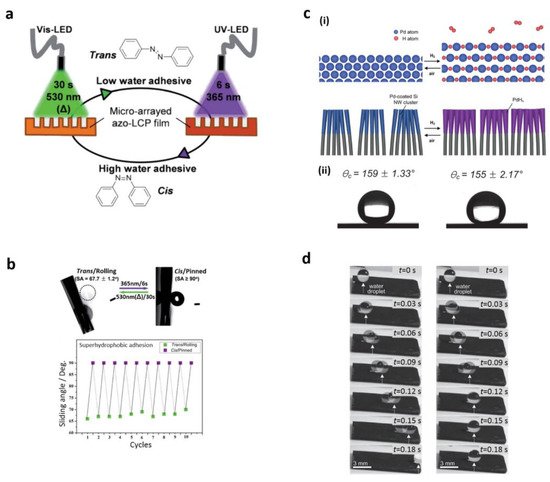
1.4. Lab-on-a-Chip Based on Open Channel Droplets
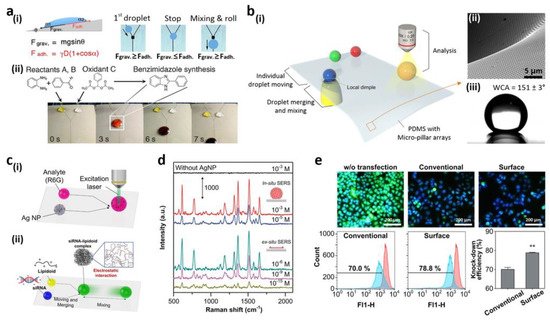
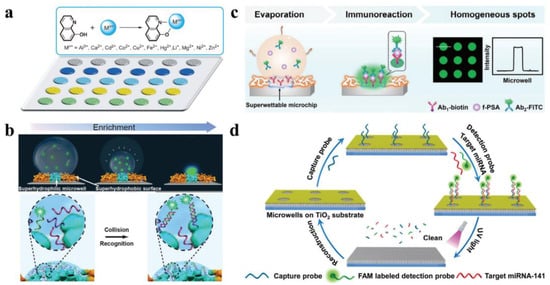
1.5. Fluorescent Intelligence Sensing Based on Bioinspired Super-Wettable Patterns
| Polymeric Materials | Fabrication Method | Application | Wetting Property | Reference |
|---|---|---|---|---|
| Hyaluronic acid (HA) | Phase separation | Tissue engineering | Hydrophobic | Ref [20] |
| Polyethylene (PE) | Shrink-induced mold method |
Antibacterial | Superhydrophobic | Ref [47] |
| Polycarbonate (PC) | Shrink-induced mold method |
Antibacterial | Superhydrophobic | Ref [47] |
| Polystyrene (PS) | Electrohydrodynamics (EHD) | Tissue engineering | Superhydrophobic | Ref [79] |
| 1H,1H,2H,2H-perfluoro-decyl trichlorosilane (PFDTS) | Wet chemical self-assembly |
Conductive stainless steel | Superhydrophobic | Ref [80] |
| Poly(vinyl alcohol) (PVA) | Solvent evaporation | Petal effects | Hydrophilic-Superhydrophobic | Ref [81] |
| Poly(p-xylylene) (PPX) | Soft lithography | Self-cleaning | Superhydrophobic | Ref [82] |
| Polydimethyl siloxane (PDMS) | Soft lithography | Water adhesion | Hydrophilic-Superhydrophobic | Ref [82] |
| Polydopamine (PD) | Soft lithography | Water adhesion | Hydrophilic-Superhydrophobic | Ref [82] |
| Polytetrafluoroethylene (PTFE) | Sol-gel strategy | Water adhesion | Superhydrophobic | Ref [69] |
| Polyvinylpyrrolidone (PVP) | Sol-gel strategy | Self-cleaning | Superhydrophobic | Ref [69] |
| Polyamide (PA) | Dip-coating method | Water and oil separation | Superhydrophobic | Ref [74] |
| Silane-modified polymer (SMP) | Spray-coating method | Water and oil separation | Superhydrophobic | Ref [74] |
This entry is adapted from the peer-reviewed paper 10.3390/polym14020238
References
- Geyer, F.; Ueda, E.; Liebel, U.; Grau, N.; Levkin, P. Superhydrophobic-superhydrophilic micropatterning: Towards genome-on-a-chip cell microarrays. Angew. Chem. Int. Ed. 2011, 50, 8424–8427.
- Piret, G.; Galopin, E.; Coffinier, Y.; Boukherroub, R.; Legrand, D.; Slomianny, C. Culture of mammalian cells on patterned superhydrophilic/superhydrophobic silicon nanowire arrays. Soft Matter 2011, 7, 8642–8649.
- Alves, N.M.; Shi, J.; Oramas, E.; Santos, J.; Tomás, H.; Mano, J.F. Bioinspired superhydrophobic poly(l-lactic acid) surfaces control bone marrow derived cells adhesion and proliferation. J. Biomed. Mater. Res. A 2009, 9, 480–488.
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941.
- Ballester Beltran, J.; Rico, P.; Moratal, D.; Song, W.; Mano, J.F. Role of superhydrophobicity in the biological activity of fibronectin at the cell-material interface. Soft Matter 2011, 7, 10803–10811.
- Auad, P.; Ueda, E.; Levkin, P.A. Facile and multiple replication of superhydrophilic-superhydrophobic patterns using adhesive tape. ACS Appl. Mater. Interfaces 2013, 5, 8053–8057.
- Efremov, A.N.; Stanganello, E.; Welle, A.; Scholpp, S.; Levkin, P.A. Micropatterned superhydrophobic structures for the simultaneous culture of multiple cell types and the study of cell-cell communication. Biomaterials 2013, 34, 1757–1763.
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863.
- Li, H.; Yang, Q.; Li, G.; Li, M.; Wang, S.; Song, Y. Splitting a droplet for femtoliter liquid patterns and single cell isolation. ACS Appl. Mater. Interfaces 2015, 7, 9060–9065.
- Galopin, E.; Piret, G.; Szunerits, S.; Lequette, Y.; Faille, C.; Boukherroub, R. Selective adhesion of bacillus cereus spores on heterogeneously wetted silicon nanowires. Langmuir 2010, 26, 3479–3484.
- Dar, A.A.; Hussain, S.; Dutta, D.; Iyer, P.K.; Khan, A.T. One-pot synthesis of functionalized 4-hydroxy-3-thiomethylcoumarins: Detection and discrimination of Co2+ and Ni2+ ions. RSC Adv. 2015, 5, 57749–57756.
- Marcon, L.; Addad, A.; Coffinier, Y.; Boukherroub, R. Cell micropatterning on superhydrophobic diamond nanowires. Acta Biomater. 2013, 9, 4585–4591.
- Neto, A.I.; Correia, C.R.; Custódio, C.A.; Mano, J. Biomimetic miniaturized platform able to sustain arrays of liquid droplets for high-throughput combinatorial tests. Adv. Funct. Mater. 2014, 24, 5096–5103.
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of cell adhesive behaviors on superhydrophobic superhydrophilic and micropatterned superhydrophobic/superhydrophilic surfaces to their surface chemistry. Langmuir 2010, 26, 8147–8154.
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592.
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845.
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part C Methods 2010, 16, 735–749.
- Meli, L.; Jordan, E.T.; Clark, D.S.; Linhardt, R.J.; Dordick, J.S. Influence of a three-dimensional, microarray environment on human cell culture in drug screening systems. Biomaterials 2012, 33, 9087–9096.
- Breslin, S.; Driscoll, L. Three-dimensional cell culture the missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249.
- Salgado, C.L.; Oliveira, M.B.; Mano, J.F. Combinatorial cell-3D biomaterials cytocompatibility screening for tissue engineering using bioinspired superhydrophobic substrates. Integr. Biol. 2012, 4, 318–327.
- Tofilon, P.J.; Buckley, N.; Deen, D.F. Effect of cell-cell interactions on drug sensitivity and growth of drug-sensitive and -resistant tumor cells in spheroids. Science 1984, 226, 862–864.
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729.
- Torisawa, Y.; Takagi, A.; Nashimoto, Y.; Yasukawa, T.; Shiku, H.; Matsue, T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials 2007, 28, 559–566.
- Brophy, C.M.; Luebke-Wheeler, J.; Amiot, B.P.; Khan, H.; Remmel, R.P.; Rinaldo, P.; Nyberg, S.L. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology 2009, 49, 578–586.
- Hsiao, A.Y.; Torisawa, Y.; Tung, Y.C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of pc-3 prostate cancer co-culture spheroids. Biomaterials 2009, 30, 3020–3027.
- Wong, S.F.; No, D.Y.; Choi, Y.Y.; Kim, D.S.; Chung, B.G.; Lee, S.H. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 2011, 32, 8087–8096.
- Lin, R.Z.; Chang, H.Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184.
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384-hanging drop array. Analyst 2011, 136, 473–478.
- Cavnar, S.P.; Salomonsson, E.; Luker, K.E.; Luker, G.D.; Takayama, S. Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids. J. Lab. Autom. 2014, 19, 208–214.
- Lee, M.; Yang, K.; Hwang, Y.H.; Byun, Y.; Lee, D.Y.; Cho, S.W.; Lee, H. Spheroform: Therapeutic spheroid-forming nanotextured surfaces inspired by desert beetle physosterna cribripes. Adv. Healthc. Mater. 2015, 4, 511–515.
- Seo, J.; Lee, J.S.; Lee, K.; Kim, D.; Yang, K.; Shin, S.; Mahata, C.; Jung, H.B.; Lee, W.; Cho, S.W.; et al. Switchable water-adhesive, superhydrophobic palladium-layered silicon nanowires potentiate the angiogenic efficacy of human stem cell spheroids. Adv. Mater. 2014, 26, 7043–7050.
- Heinonen, S.; Huttunen Saarivirta, E.; Nikkanen, J.P.; Raulio, M.; Priha, O.; Laakso, J.; Storgårds, E.; Levänen, E. Antibacterial properties and chemical stability of superhydrophobic silver-containing surface produced by sol-gel route. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 149–161.
- Wang, Z.; Ou, J.; Wang, Y.; Xue, M.; Wang, F.; Pan, B.; Li, C.; Li, W. Anti-bacterial superhydrophobic silver on diverse substrates based on the mussel-inspired polydopamine. Surf. Coat. Technol. 2015, 280, 378–383.
- Heinonen, S.; Nikkanen, J.P.; Laakso, J.; Raulio, M.; Priha, O.; Levänen, E. Bacterial growth on a superhydrophobic surface containing silver nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012064.
- Berendjchi, A.; Khajavi, R.; Yazdanshenas, M. Fabrication of superhydrophobic and antibacterial surface on cotton fabric by doped silica-based sols with nanoparticles of copper. Nanoscale Res. Lett. 2011, 6, 594.
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-nanoparticle-colored cotton fabrics with tunable colors and durable antibacterial and self-healing superhydrophobic properties. Adv. Funct. Mater. 2016, 26, 569–576.
- Khalil-Abad, M.S.; Yazdanshenas, M.E. Superhydrophobic antibacterial cotton textiles. J. Colloid Interface Sci. 2010, 351, 293–298.
- Zhang, L.; Zhang, L.; Yang, Y.; Zhang, W.; Lv, H.; Yang, F.; Lin, C.; Tang, P. Inhibitory effect of super-hydrophobicity on silver release and antibacterial properties of super-hydrophobic Ag/TiO2 nanotubes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1004–1012.
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platform. Macromolecules 2009, 42, 8573–8586.
- Song, K.; Gao, A.; Cheng, X.; Xie, K. Preparation of the superhydrophobic nano-hybrid membrane containing carbon nanotube based on chitosan and its antibacterial activity. Carbohydr. Polym. 2015, 130, 381–387.
- Yang, H.; You, W.; Shen, Q.; Wang, X.; Sheng, J.; Cheng, D.; Cao, X.; Wu, C. Preparation of lotus-leaf-like antibacterial film based on mesoporous silica microcapsule-supported Ag nanoparticles. RSC Adv. 2014, 4, 2793–2796.
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601.
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019.
- Liu, T.; Dong, L.; Liu, T.; Yin, Y. Investigations on reducing microbiologically influenced corrosion of aluminum by using super-hydrophobic surfaces. Electrochim. Acta 2010, 55, 5281–5285.
- Ye, W.; Shi, Q.; Hou, J.; Jin, J.; Fan, Q.; Wong, S.; Xu, X.; Yin, J. Superhydrophobic coating of elastomer on different substrates using a liquid template to construct a biocompatible and antibacterial surface. J. Mater. Chem. B 2014, 2, 7186–7191.
- Grinthal, A.; Aizenberg, J. Mobile interfaces, Liquids as a perfect structural material for multifunctional, antifouling surfaces. Chem. Mater. 2014, 26, 698–708.
- Freschauf, L.R.; McLane, J.; Sharma, H.; Khine, M. Shrink-induced superhydrophobic and antibacterial surfaces in consumer plastics. PLoS ONE 2012, 7, e40987.
- Epstein, A.K.; Wong, T.S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187.
- Leslie, D.C.; Waterhouse, A.; Berthet, J.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140.
- Balu, B.; Berry, A.D.; Hess, D.W.; Breedveld, V. Patterning of superhydrophobic paper to control the mobility of micro-liter drops for two-dimensional lab-on-paper applications. Lab Chip 2009, 9, 3066–3075.
- Mertaniemi, H.; Jokinen, V.; Sainiemi, L.; Franssila, S.; Marmur, A.; Ikkala, O.; Ras, R.H.A. Superhydrophobic tracks for low-friction, guided transport of water droplets. Adv. Mater. 2011, 23, 2911–2914.
- You, I.; Kang, S.M.; Lee, S.; Cho, Y.O.; Kim, J.B.; Lee, S.B.; Nam, Y.S.; Lee, H. Polydopamine microfluidic system toward a two-dimensional, gravity-driven mixing device. Angew. Chem. Int. Ed. 2012, 51, 6126–6130.
- Sousa, M.P.; Mano, J.F. Superhydrophobic paper in the development of disposable labware and lab-on-paper devices. ACS Appl. Mater. Interfaces 2013, 5, 3731–3737.
- Elsharkawy, M.; Schutzius, T.M.; Megaridis, C.M. Inkjet patterned superhydrophobic paper for open-air surface microfluidic devices. Lab Chip 2014, 14, 1168–1175.
- Zhao, Y.; Xu, Z.; Niu, H.; Wang, X.; Lin, T. Magnetic liquid marbles, Toward “lab in a droplet”. Adv. Funct. Mater. 2015, 25, 437–444.
- Kim, D.; Seo, J.; Shin, S.; Lee, S.; Lee, K.; Cho, H.; Shim, W.; Lee, H.; Lee, T. Reversible liquid adhesion switching of superamphiphobic Pd-decorated Ag dendrites via gas-induced structural changes. Chem. Mater. 2015, 27, 4964–4971.
- Jonsson Niedziolka, M.; Lapierre, F.; Coffinier, Y.; Parry, S.J.; Zoueshtiagh, F.; Foat, T.; Thomy, V.; Boukherroub, R. EWOD driven cleaning of bioparticles on hydrophobic and superhydrophobic surfaces. Lab Chip 2011, 11, 490–496.
- Lapierre, F.; Piret, G.; Drobecq, H.; Melnyk, O.; Coffinier, Y.; Thomy, V.; Boukherroub, R. High sensitive matrix-free mass spectrometry analysis of peptides using silicon nanowires-based digital microfluidic device. Lab Chip 2011, 11, 1620–1628.
- Lapierre, F.; Harnois, M.; Coffinier, Y.; Boukherroub, R.; Thomy, V. Split and flow: Reconfigurable capillary connection for digital microfluidic devices. Lab Chip 2014, 14, 3589–3593.
- Li, L.; Breedveld, V.; Hess, D.W. Hysteresis controlled water droplet splitting on superhydrophobic paper. Colloid Polym. Sci. 2013, 291, 417–426.
- You, I.; Lee, T.G.; Nam, Y.S.; Lee, H. Fabrication of a micro-omnifluidic device by omniphilic/omniphobic patterning on nanostructured surfaces. ACS Nano 2014, 8, 9016–9024.
- Seo, J.; Lee, S.K.; Lee, J.; Seung, L.J.; Kwon, H.; Cho, S.W.; Ahn, J.H.; Lee, T. Path-programmable water droplet manipulations on an adhesion controlled superhydrophobic surface. Sci. Rep. 2015, 5, 12326.
- Huang, Y.; Li, F.; Qin, M.; Jiang, L.; Song, Y. A multi-stopband photonic-crystal microchip for high-performance metal-ion recognition based on fluorescent detection. Angew. Chem. Int. Ed. Engl. 2013, 52, 7296–7299.
- Xu, L.P.; Chen, Y.; Yang, G.; Shi, W.; Dai, B.; Li, G.; Cao, Y.; Wen, Y.; Zhang, X. Ultratrace DNA Detection Based on the Condensing-Enrichment Effect of Superwettable Microchips. Adv. Mater. 2015, 27, 6878–6884.
- Chen, Y.; Xu, L.P.; Meng, J. Superwettable microchips with improved spot homogeneity toward sensitive biosensing. Biosens. Bioelectron. 2018, 102, 418–424.
- Wu, T.; Xu, T.; Chen, Y.; Yang, Y.; Xu, L.; Zhang, X.; Wang, S. Renewable superwettable biochip for miRNA detection. Sens. Actuators B 2018, 258, 715–721.
- Chen, X.; Liu, C.; Xu, Z.; Pan, Y.; Liu, J.; Du, L. An effective PDMS microfluidic chip for chemiluminescence detection of cobalt (II) in water. Microsyst. Technol. 2012, 19, 99–103.
- Sima, F.; Xu, J.; Wu, D.; Sugioka, K. Ultrafast laser fabrication of functional biochips: New avenues for exploring 3D micro- and nano-environments. Micromachines 2017, 8, 40.
- He, J.; Zhao, Y.; Yuan, M.; Hou, L.; Abbas, A.; Xue, M.; Ma, X.; He, J.; Qu, M. Fabrication of durable polytetrafluoroethylene superhydrophobic materials with recyclable and self-cleaning properties on various substrates. J. Coat. Technol. Res. 2020, 17, 755–763.
- He, J.; Zhang, Y.; Zhou, Y.; Wang, J.; Zhao, Y.; Ma, L.; Abbas, A.; Qu, M. A facile approach to fabricate the durable and buoyant superhydrophobic fabric for efficient oil/water separation. Fibers Polym. 2019, 20, 1003–1010.
- Stetsyshyn, Y.; Raczkowska, J.; Budkowski, A.; Awsiuk, K.; Kostruba, A.; Nastyshyn, S.; Harhay, K.; Lychkovskyy, E.; Ohar, H.; Nastishin, Y. Cholesterol-based grafted polymer brushes as alignment coating with temperature-tuned anchoring for nematic liquid crystals. Langmuir 2016, 32, 11029–11038.
- Pan, Z.; Cheng, F.; Zhao, B. Bioinspired polymeric structures with special wettability and their applications. Polymers 2017, 9, 725.
- Raczkowskaa, J.; Stetsyshynb, Y.; Awsiuka, K.; Lekkac, M.; Marzec, M.; Harhay, K.; Ohar, H.; Ostapiv, D.; Sharand, M.; Yaremchuk, I.; et al. Temperature-responsive grafted polymer brushes obtained from renewable sources with potential application as substrates for tissue engineering. Appl. Surf. Sci. 2017, 407, 546–554.
- Zhang, T.; Wang, S.; Huang, J.; Jin, Y.; Zhao, G.; Zhang, C.; Li, C.; Yu, J.; Jia, Y.; Jiao, F. Facile fabrication of versatile superhydrophobic coating for efficient oil/water separation. J. Dispers. Sci. Technol. 2021, 42, 363–372.
- Wang, R.; Wu, F.; Yu, F.; Zhu, J.; Gao, X.; Jiang, L. Anti-vapor-penetration and condensate microdrop self-transport of superhydrophobic oblique nanowire surface under high subcooling. Nano Res. 2021, 14, 1429–1434.
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59.
- Zeng, J.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.; Chen, Y.; Huang, J.; Wang, D.; Yan, Z.; Yin, Y. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 2020, 32, 100855.
- Xu, T.; Xu, L.-P.; Zhang, X.; Wang, S. Bioinspired superwettable micropatterns for biosensing. Chem. Soc. Rev. 2019, 48, 3153–3165.
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. Int. Ed. 2004, 116, 4438–4441.
- Li, Y.; Huang, X.J.; Heo, S.H.; Li, C.C.; Choi, Y.K.; Cai, W.P.; Cho, S.O. Superhydrophobic bionic surfaces with hierarchical microsphere/SWCNT composite arrays. Langmuir 2007, 23, 2169–2174.
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119.
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404.
