Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Protein-tyrosine phosphatase 1B (PTP1B) negatively regulates insulin signaling pathways and plays an important role in type 2 diabetes mellitus (T2DM), as its overexpression may induce insulin resistance.
- protein-tyrosine phosphatase 1B
- type 2 diabetes mellitus
- insulin signaling pathways
1. Introduction
Diabetes mellitus (DM) is considered a major health problem worldwide [1]. Obesity and diabetes incidence still continue increasing due to globalization, mechanization, and changes in human lifestyle and daily routines [2]. According to the International Diabetes Federation (IDF), it was estimated that in 2017 there were 451 million (age 18–99 years) people with diabetes worldwide. These figures were expected to increase to 693 million by 2045 [3]. DM is a chronic metabolic disease that results from defects in insulin action, insulin secretion, or both, leading to persistent hyperglycemia [4].
Currently, type 2 diabetes mellitus (T2DM) represents a major threat to health [5]. Characterized by increased blood glucose levels, this is the underlying reason for several complications, including cardiovascular disorders, blindness, kidney failure, and peripheral nerve damages [6]. The development of T2DM and its complications are related, in most cases, to insulin resistance and postprandial hyperglycemic variations [7,8]. Thus, an effective drug for controlling insulin resistance may be beneficial in improving the quality life of T2DM patients. Several pharmacological strategies have been investigated on DM treatment, including insulin release stimulation, gluconeogenesis inhibition, glucose transport activity increase, and intestinal glucose absorption reduction [9]. Insulin supplements and other oral anti-diabetic drugs can be used alone or in combination to improve glycemic regulation [10]. However, some of the available anti-diabetic drugs have either the disadvantage of having low efficacy or serious side effects [11]. Thus, there is a continuous search for more effective and safer anti-hyperglycemic agents, especially from natural origins.
Insulin sensitizers, such as thiazolidinediones (TZDs or glitazones) have been used as effective drugs for T2DM treatment [12]. The identification of the enzyme responsible for the dephosphorylation of insulin receptors, called protein-tyrosine phosphatase 1B (PTP1B), showed that the inhibitors of such an enzyme could be employed as insulin sensitizer agents and, therefore, as promising anti-diabetic drugs [13]. This hypothesis was confirmed in mouse models, where it was found that PTP1B gene disruption can increase insulin sensitivity. Similar results were also obtained when PTP1B antisense nucleotides suppressed PTP1B gene expression [14].
Protein tyrosine phosphatases (PTPs) constitute a huge and structurally variable family of highly regulated enzymes. Most PTPs have been proposed to be targets for advanced drug discovery, and PTP1B is one of the well-established enzymes among PTPs [15,16,17]. It was the first isolated member of the PTP superfamily, and since then, growing evidence has linked it with insulin resistance, obesity, and T2DM. Numerous studies have shown that PTP1B can negatively regulate insulin and leptin signaling pathways. Indeed, PTP1B dephosphorylates both insulin receptor and its substrate IRS-1 in the insulin signaling pathway [18,19], whereas in the leptin pathway, PTP1B binds and dephosphorylates tyrosine kinase downstream of the Janus-Activated Kinase 2 (JAK2) leptin receptor [20]. In cell cultures, PTP1B overexpression causes a decrease in the insulin-stimulated phosphorylation of IR and IRS-1, while PTP1B raises insulin-initiated signaling level reduction [21,22]. The hypothesis that PTP1B expression can contribute to diabetes and obesity is supported by quantitative analysis of trait loci and mutations in the human PTP1B gene [23]. In in vivo studies, PTP1B knockout mice exhibited elevated resistance to high-fat diet-induced obesity and insulin sensitivity [24,25]. In addition, other studies on tissue-specific PTP1B knockout mice have shown that leptin action, adiposity, as well as body weight are controlled by neuronal PTP1B [26]. Generally, many studies suggest that PTP1B inhibitors constitute a highly promising approach for T2DM and obesity amelioration.
Aryl carboxylic acids, such as isoxazole [27], hydroxylpropionic [28], 2-oxalylamino benzoic (OBA) acids [29], and thiophene diacid [30], have been recognized as alternative phosphotyrosine (pTyr) surrogates to overcome the lack of cellular activity of highly charged phosphonates (Figure 1). Furthermore, it was reported that benzyl aryl α-ketoacid derivatives revealed significant PTP1B inhibitory effects in a non-competitive pattern, targeting conserved protein loop (WPD loop) open conformation [31]. It was also noticed that the existence of a benzyl group in these bioactive molecules may enhance PTP1B binding affinity, and being hydrophobic in nature, it also increases their cell membrane permeability. Recent studies also suggested that PTP1B may become an oncogene in breast cancer [16]. Accordingly, multiple studies have been conducted focusing on the development of new PTP1B inhibitors for the treatment of T2DM, obesity, and cancer, but to the authors’ knowledge there are no review articles published on this subject. In this sense, the present review aims to provide an overview of the role of PTP1B in T2DM insulin signaling and treatment, and to highlight the most recent findings on several compounds and extracts discovery from marine organisms and their relevance as upcoming PTP1B inhibitors.
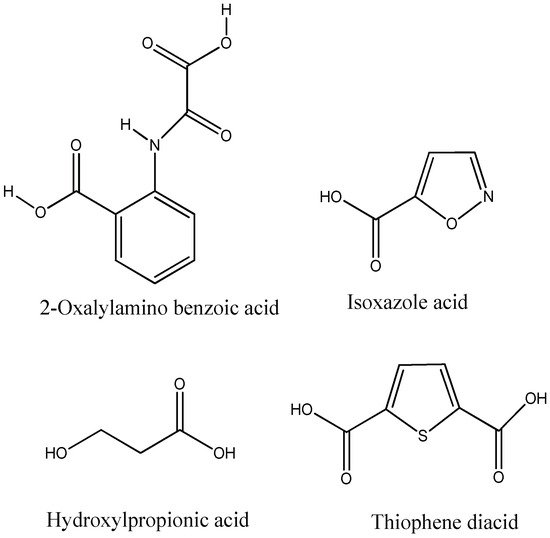
Figure 1. Structures of phosphotyrosine (pTyr) surrogate acids.
2. Marine Sources as Upcoming Therapeutic Agents
The marine environment is considered a wide and relatively unexploited source of bioactive compounds with high biodiversity, including fatty acids (especially polyunsaturated fatty acids), proteins, polyphenols, sterols, sulfated polysaccharides, and pigments [32,33,34,35,36]. Indeed, marine algae has been increasingly exploited as renowned sources of metabolites with promising biological effects, including antioxidant, hypoglicemic, hypotensive, hypolipidemic, antibacterial, and antiviral activities [37,38]. Specifically, macroalgae are considered healthy foods as they are rich in minerals and dietary fibers. Traditionally, the Far East and Hawaiian Islands, Japan, Korea, and China consume marine algae as a common component of their diets. Macroalgae species can reach 9000 species and can be classified according to their pigment composition into three classes, i.e., Phaeophyta, Rhodophyta, and Chlorophyta (also known as brown, red, and green algae, respectively) [39].
Unique metabolites from diverse classes have been isolated from different marine plants, with in vivo remarkable pharmacological effects [40], such as anticancer, anti-hyperlipidemic, anti-diabetic, anti-hypertensive, antioxidant, anti-inflammatory, anticoagulant, anti-estrogenic, antibacterial, antifungal, antiviral, immunomodulatory, neuroprotective, and tissue healing properties [41]. More recently, as a result of the characterization of a large number of bioactive metabolites from marine macroalgae, there has been a growing interest in the search for potential applications of macroalgae and their metabolites as functional constituents for human and animal health benefits [42]. Functional constituents of macroalgae have been increasingly used as food supplements as well as for anti-diabetic purposes [40]. Hereby, the possible applications of marine macroalgae and/or macroalgae-derived bioactive metabolites for PTP1B inhibitory effects have been greatly expanded.
This entry is adapted from the peer-reviewed paper 10.3390/molecules23123334
This entry is offline, you can click here to edit this entry!
