Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Fentanyl is known as a synthetic narcotic analgesic, which can act as an agonist of opioid receptors, being about 100 times more potent than heroin or morphine.
- fentanyl
- fentanyl analogs
- amperometry
- voltammetry
- metabolite
- oxidation
1. Fentanyl
Fentanyl and its analogs with piperidine-based structures have significantly different structures than morphine and other semi-synthetic opioids (e.g., codeine, hydrocodone, oxycodone, buprenorphine, methadone, etc.). Moreover, it has fewer adverse effects than morphine or pethidine [1]. Thanks to these advantages and relatively small dosages needed, they are often used for anesthesia (namely in surgical settings), treatment of (chronic) pains, and supplemental medications for breakthrough pain in cancer patients. In the average person, anesthesia is achieved after 25–125 mg of fentanyl [2]. Moreover, fentanyls stabilize cardiovascular activity (even in patients under critical conditions [3]), increasing their medical use. Fentanyl belongs to the most potent opioids available for human medical use [1].
Fentanyl is commercially available as a water-soluble drug in hydrochloride or citrate form [3]. According to various literature sources, fentanyl dissociation constant pKA amounts to between 8.4 and 9.0 (Table 1), thus making it partially unionized in blood and bound to specific compounds, such as erythrocytes, albumins, and other endogenous compounds. These values are relatively close to those of morphine (pKA = 8.08) or fentanyl analog sufentanil (pKA = 8.51) [4]. Fentanyl is highly lipophilic (logP 2.3 octanol/buffer pH 7.4 [5]) and can enter the central nervous system (CNS) 100 times more easily than morphine [3]. Unfortunately, it has also been used since the 70’s as an illicit street drug individually or in a mixture with other illicit drugs, such as heroin or synthetic cannabinoids.
Table 1. Physicochemical parameters of the most important fentanyl analogs.
| Compound | Mol. Mass [g mol−1] |
Dissociation Constant pKA | PARTITION Coefficient Log P | Solubility in Water [g L−1] |
|---|---|---|---|---|
| Fentanyl | 336.471 | 8.99 (DB, e) | 4.05 (DB, e) | 0.74 (DB, e) |
| 8.4 [6] | 4.12 (DB, p-AG) | 0.15 (p-SF) | ||
| 8.92 ± 0.20 (p-SF) | 3.82 (DB, p-CA) | |||
| 8.99 [4] | 3.683 (p-SF) | |||
| 8.44 ± 0.05 [7] | 2.3 (pH 7.4 [5]) | |||
| 8.43 [8] | ||||
| Norfentanyl | 232.321 | 9.81 ± 0.10 (p-SF) | 1.59 (CS, p-ACD/LogP) | 7.4 (p-SF) |
| 1.667 (p-SF) | ||||
| Sufentanil | 386.552 | 8.86 (DB, p-SF) | 3.95 (DB, e) | 0.076 [9] |
| 8.51 [4] | 3.4 (DB, p-AG) | 0.012 (DB, p) | ||
| 8.01 [9] | 3.61 (DB, p-CA) | 0.15 (p-SF) | ||
| 8.0 [6] | 3.950 (p-SF) | |||
| 7.89 ± 0.20 (p-SF) | ||||
| Carfentanyl | 394.515 | 8.05 (DB, p-CA) | 3.7 (DB, p-AG) | 0.0259 (DB, p-AG) |
| 7.76 ± 0.20 (p-SF) | 3.67 (DB, p-CA) | 0.19 (p-SF) | ||
| 3.684 (p-SF) | ||||
| Acetylfentanyl | 322.44 | 8.92 ± 0.10 (p-SF) | 3.173 (p-SF) | 0.30 (p-SF) |
| alfa-methylfentanyl | 350.50 | 9.37 ± 0.20 (p-SF) | 4.49 (DB, p-AG) | 0.014 (DB, p-AG) |
| 4.23 (DB, p-CA) | ||||
| Acrylfentanyl | 334.45 | 8.72 ± 0.10 (p-SF) | 3.201 (p-SF) | 0.037 (p-SF) |
| Butyrfentanyl | 350.50 | 8.92 ± 0.20 (p-SF) | 4.44 (DB, p-AG) | 0.0137 (DB, p-AG) |
| 4.26 (DB, p-CA) | ||||
| Cyclopropylfentanyl | 348.48 | 8.75 ± 0.10 (p-SF) | 3.564 (p-SF) | 0.045 (p-SF) |
| Furanylfentanyl | 374.48 | 8.71 ± 0.10 (p-SF) | 5.277 (p-SF) | 0.012 (p-SF) |
| Methoxyacetylfentanyl | 352.47 | 8.88 ± 0.20 (p-SF) | 2.574 (p-SF) | 0.85 (p-SF) |
| Ocfentanyl | 370.46 | 8.81 ± 0.20 (p-SF) | 2.816 (p-SF) | 0.26 (p-SF) |
| tetrahydrofuranylfentanyl | 378.51 | 8.71 ± 0.10 (p-SF) | 2.815 (p-SF) | 0.016 (p-SF) |
| p-fluroisobutyrylfentanyl | 368.49 | 8.91 ± 0.20 (p-SF) | 4.150 (p-SF) | 0.027 (p-SF) |
| Alfentanil | 416.52 | 7.82 ± 0.20 (p-SF) | 2.16 (DB, e) | 0.252 (DB, p-AG) |
| 2.2 (DB, p-AG) | ||||
| 2.81 (DB, p-CA) | ||||
| Remifentanil | 376.45 | 6.65 ± 0.20 (p-SF) | 1.75 (DB, p-AG) | 0.591 (DB, p-AG) |
| 1.52 (DB, p-CA) |
DB = DrugBank (DrugBank, Edmonton, Alberta, USA), CS = ChemSpider (Royal Society of Chemistry, UK), PC = PubChem (National Center for Biotechnology Information, USA), SF = SciFinder (pKa—the most basic; 25 °C) (American Chemical Society, Washington, D.C., USA) e—experimental value, p—predicted value, CA—ChemAxon (ChemAxon, Budapest, Hungary), AG—ALOGPS (Helmholtz Zentrum München, Munich, Germany), ACD/LogP—Advanced Chemistry Development software/LogP (Advanced Chemistry Development, Toronto, Canada). p-SF: Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2020 ACD/Labs) (Advanced Chemistry Development, Toronto, ON, Canada).
Fentanyl analogs were synthesized to develop new opioid drugs with better pharmacological properties and fewer side effects. Despite a large number of available fentanyl analogs, only three have been approved for human medical use, i.e., sufentanil, alfentanil, and remifentanil [1][10]. The administration is limited to intravenous anesthesia or severe pain treatment. Compared to fentanyl, its analogs may have slightly different metabolic pathways, physiological activity, and properties. For example, alfentanil has a smaller volume of distribution than fentanyl, less solubility in lipids, and a shorter elimination half-life [11]. As a result, it has lower potency than fentanyl and has been widely used in medicine [12]. Sufentanil was reported as 5–10 times more potent than fentanyl, and its interactions are more rapid [12]. Another one, carfentanyl, has been approved for veterinary use in the case of large animals [1][12]. Carfentanyl is 10,000 times more potent than morphine as an analgesic.
Physicochemical parameters of fentanyl and its chosen derivatives (used in human and veterinary medicine or misused as illicit drugs) are summarized in Table 1.
Many other fentanyl derivatives have been described since the 1960s, but they have not been used in human or veterinary medicine. The new non-therapeutic fentanyl analogs have been later described as NPSs or NSOs, seriously affecting the neurological system [10]. New fentanyl analogs are reported from year to year; for example, three of them appeared on the European market in 2018: 3-methylcrotonylfentanyl, furanylbenzylfentanyl, and 4-fluorocyclopropylbenzylfentanyl [13].
There are many non-therapeutic fentanyl analogs reported to the United Nations Office on Drugs and Crime Early Warning Advisory (UNODC EWA), which are common in Europe, Asia, and America [1][11][13]. The United States Drug Enforcement Agency (DEA) listed fentanyl and its derivatives (isomers, thioethers, and salts) in the Schedule I list [14]. The most popular fentanyl analogs in 2012–2018 used either for medical treatment or appeared as illicit street drugs. Metabolism and toxicological aspects of fentanyl and other NSOs derived from fentanyl are discussed below.
2. Metabolism of Fentanyl and Fentanyl Analogs
The knowledge of the metabolism of fentanyl and its analogs is essential for the identification of administered substance(s) in various body fluids ante- and post-mortem (namely blood, plasma, serum, urine, cerebrospinal fluid, vitreous humor, and organs) after acute or chronic intoxication or death. The study of these processes is critical due to their short biological half-life (units of hours in case of oral or intravenous applications) and consequently the short detection window of the parent fentanyl(s) in the blood. Identification of characteristic metabolites, first of all in urine, may contribute to the exact identification of parent substance(s).
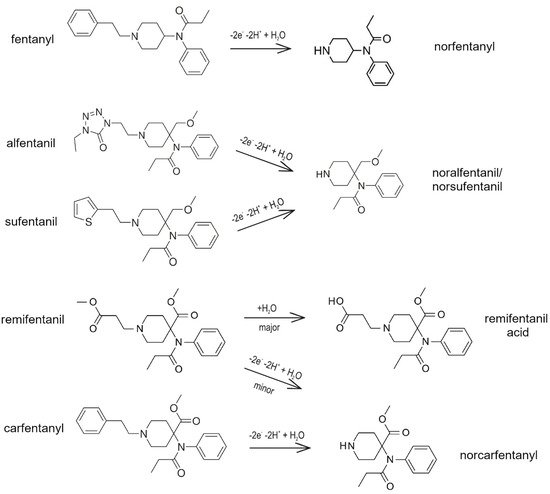
While metabolites of morphine are pharmacologically active, metabolites of fentanyl and medically-approved fentanyl derivatives are mostly inactive [15]. Fentanyl has several sites, which can take part in its metabolic transformation. Fentanyl is metabolized mainly in the human liver to the main metabolite norfentanyl (26–55% of fentanyl dose is excreted as norfentanyl). This principal metabolic pathway is caused by cytochrome CYP3A4 [15], together with CYP3A5 and CYP3A7 isoenzymes, through the oxidative N-dealkylation of the fentanyl’s piperidine ring [16]. The same N-dealkylation takes place in duodenal microsomes [17][11]. Other possible metabolic reactions transform fentanyl into hydroxyfentanyl, hydroxynorfentanyl, and despropionylfentanyl. These metabolites can take part in the subsequent biotransformation at enzyme catechol-O-methyltransferase to the secondary metabolic products [11]. Other metabolic pathways change fentanyl into hydroxypropionylfentanyl, hydroxypropionylnorfentanyl, or despropionylfentanyl. Metabolites are mainly present in saliva, urine, stool, and human plasma [11]. About 70% of the administered dose is excreted in the urine in 72 h (mostly in the form of metabolites), and about 10 to 20% of the administered dose is excreted unchanged in 48 h. Only 8–10% of unchanged fentanyl is released from the body through the renal or fecal pathway [17]. Fentanyl crosses the placenta, and small amounts may be found in breast milk, too.
As mentioned above, three fentanyl analogs (alfentanil, sufentanil, and remifentanil) are widely used in anesthesia and pain treatment. Alfentanil and sufentanil are metabolized similarly to fentanyl via the hepatic pathway to identical N-dealkylated products (norsufentanil, noralfentanil). From this point of view, the administered alfentanil or sufentanil cannot be distinguished using the methods which detect products of metabolic transformation only [17][11]. Norsufentanil exhibits some bioactivity, the other product of sufentanil metabolism demonstrates about 10% of the original sufentanil activity only, which is too small to be clinically significant [11].
Remifentanil is mostly metabolized directly in the blood by non-specific esterases located in erythrocytes (95%) and has a very short time of activity [18][19]. Its main metabolite, remifentanil acid, is practically non-active and is removed from the body via the renal pathway with an elimination half-life of approximately 90 min [17][11][18][19]. It is the only analog that is metabolized by non-CYP enzymes [17].
Carfentanyl is one of the well-known fentanyl analogs used in veterinary medicine, which shows potency 30–100 times higher than fentanyl itself. According to our literary research, there has not been a published study about metabolic pathways of this drug in vivo in humans. Identification of twelve metabolites of carfentanyl was done using human liver microsomes and human hepatocytes [20][11][21]. N-demethylation as the main biotransformation was predicted in silico and confirmed by high-resolution mass spectrometry. Carfentanyl and its metabolite, norfentanyl, may accumulate in the human body, which can cause resistance to the antidote [20].
Figure 1 presents the proposed mechanism of metabolism of fentanyl and its four medicinally-used analogs (alfentanil, sufentanil, and remifentanil) and veterinary medicine (carfentanyl).
Furanylfentanyl, an illegal drug, is another analog with seven times higher potency than fentanyl. Its four metabolites have been found in urine samples taken from intoxicated patients. In almost all cases, hydrolysis product 4-anilino-N-phenethylpiperidine (4-ANPP) and its sulfate conjugate were found. A unique metabolite, formed by dihydrodiol formation of the heterocyclic furanyl moiety, was observed in 86% of cases [11][23]. On the other hand, N-dealkylated metabolite norfuranylfentanyl was detected only rarely [24].
The pharmacological properties of acetylfentanyl (street names: “China town” or “Synthetic heroin”) are similar to heroin. Up to date, 32 metabolites of acetylfentanyl have been identified in vivo. The main metabolite is N-dealkylated product noracetylfentanyl. The next metabolizations of noracetylfentanyl include hydroxylation followed by glucuronidation or sulfation. Dihydroxylation was also confirmed, followed by glucuronidation or sulfation. The other metabolite reactions include monohydroxylation and carbonylation, dihydrodiol formation, dihydroxylation with methylation at the phenyl ring followed by glucuronidation or sulfation, and amide hydrolysis, followed by hydroxylation [24].
Ocfetanyl is a 200 times more potent derivative than morphine. Its biotransformation starts with O-demethylation forming the main metabolite. The following reactions include hydroxylation and glucuronidation of O-demethylated ocfentanyl.
Butyrfentanyl is 7 times more potent than morphine. The work of Staehali et al. describes two main metabolites hydroxy-butyrfentanyl and carboxybutyrfentanyl in vivo after fatal intoxication by butyrfentanyl [25].
Acrylfentanyl is abused alone or mixed with other drugs. Nor-acrylfentanyl, formed by N-dealkylation at the piperidine nitrogen, has been identified as the main metabolite of acrylfentanyl. Other metabolites have been identified, such as monohydroxy- and dihydroxy-derivatives. Monohydroxylation and dihydroxylation occurred at both the phenyl ring and the phenethyl moiety. Three glucuronides have been identified in urine samples without hydrolysis [24].
Cyclopropylfentanyl undergoes extensive metabolism. Eleven metabolites of cyclopropylfentanyl have been identified in the pooled hydrolyzed and non-hydrolyzed human urine of drug abusers. Cyclopropylfentanyl and norcyclopropylfentanyl have been detected in non-hydrolyzed urine. Other metabolites, together with norcyclopropylfentanyl, were conjugated and observed after urine hydrolysis. Cyclopropylfentanyl was detected in both samples, and hydrolyzed, as well as non-hydrolyzed, respectively. Major metabolites in the hydrolyzed samples were 4-hydroxyphenethyl cyclopropylfentanyl (mostly conjugated), 4-hydroxy-3-methoxyphenethyl cyclopropylfentanyl (mostly conjugated), phenethyl dihydrodiol cyclopropylfentanyl, and norcyclopropylfentanyl. Norcyclopropylfentanyl was the most abundant metabolite in the non-hydrolyzed samples [26].
3. Determination of Fentanyl and Analogs
As stated above, fentanyl and its analogs are commonly used as illicit drugs, especially in mixtures with heroin. Unfortunately, there are some challenges in their proper identification and determination [27]. These difficulties are caused by complicated mixtures with many interferents (frequently of similar chemical structures [28]) and a lack of available analytical standards. Moreover, fast changes in available psychoactive substances on the illicit market could be problematic [29]. To face these challenges, it is important to develop new methods of detection and determination of synthetic opioids.
Due to the high risk of fentanyl and its analogs usage in medical treatments and their abuse as illicit street drugs, there is an urgent need to develop proper determination and validation procedures, both for toxicological and medical purposes. Many different analytical methods have been introduced during the last few years to determine fentanyl and its analogs, e.g., enzyme-linked immunosorbent assay (ELISA) [30], high-performance liquid chromatography (HPLC) [29][31][32][33], or gas chromatography (GC) [29][34]. Other techniques, such as colorimetric detection, Raman, IR, or NMR spectroscopy, are described in the literature, as well [35][36][37].
Especially, chromatographic techniques coupled with mass spectrometry (MS) have been broadly used for fentanyls determination in real samples. This method is often chosen for complex real samples. Proper determination of all sample components often requires separation, selective, and sensitive detection. HPLC-MS methods meet mostly these requirements [29][31][32][33][36]. The other techniques used for the extraction of fentanyls from biological samples are solid-phase extraction (SPE) and liquid-liquid extraction (LLE). LC-MS/MS has been used as a fast and efficient technique with limits of detection (LODs) of 0.01 µg·L−1 for many fentanyl analogs [38].
Immunoassays have been used for selective determination of fentanyl and butyrfentanyl, as well, especially as a first-step analysis. It can be used even by non-qualified staff, which is important in medical and toxicological procedures [38]. The affinity of various fentanyl metabolites to the fentanyl antibody varies significantly [39]. Immunoassay test ELISA was used, e.g., for batch analysis of real samples in Forensic Toxicology Laboratory (FTL) at the Rhode Island State Health Laboratories. The samples were collected from dead humans after overdosing on illicit drugs containing fentanyl analogs. This test is very sensitive and can be performed using ELISA kits, which are readily commercially available. The drawbacks of this method are lower specificity and possible false-positive results; therefore, another method is often necessary to confirm the result [30].
For the analysis of extremely complicated biological matrices, including blood or cerebrospinal fluid, the elaborate multi-step separation methods are often irreplaceable. Unfortunately, many of the aforementioned methods are expensive, time-consuming, or require complicated instrumentation or preparation procedure [40][41]. Many other matrices, such as pharmaceutical samples, drug preparation, tap water, saliva, or even blood plasma, can be successfully analyzed using simpler methodology and instrumentation. Therefore, the aim to develop a fast, inexpensive, and simple procedure, with high efficiency and low LOD, is quite clear. The electroanalytical methods exhibit such advantages, which are attractive for medical and pharmaceutical analyses of synthetic opioids [36][40][41][42]. The possibility to modify the electrode surfaces can further improve the selectivity and sensitivity. Furthermore, electrochemical detectors can be coupled with flow systems, HPLC, or electrophoresis [36].
Funding
This review was funded by the project of the Czech Science Foundation (GA ČR) No.
20-07350S.
20-07350S.
This entry is adapted from the peer-reviewed paper 10.3390/bios12010026
References
- Peng, P.W.H.; Sandler, A.N. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 1999, 90, 576–599, https://doi.org/10.1097/00000542-199902000-00034.
- Bazley, M.M.; Logan, M.; Baxter, C.; Robertson, A.A.B.; Blanchfield, J.T. Decontamination of fentanyl and fentanyl analogues in field and laboratory settings: A review of fentanyl degradation. Aust. J. Chem. 2020, 73, 868–879, https://doi.org/10.1071/Ch19669.
- UNODC. Fentanyl and Its Analogues—50 Years on. Available online: https://www.unodc.org/documents/scientific/Global_SMART_Update_17_web.pdf (accessed on 19 October 2020).
- Roy, S.D.; Flynn, G.L. Solubility behavior of narcotic analgesics in aqueous-media–solubilities and dissociation-constants of morphine, fentanyl, and sufentanil. Pharm. Res. 1989, 6, 147–151, https://doi.org/10.1023/A:1015932610010.
- Moffat, A.C.; Osselton, M.D.; Widdop, B. Clarke's Isolation and Identification of Drugs in Pharmaceuticals, Body Fluids, and Post-Mortem Material, 4th ed.; Pharmaceutical Press: London, UK, 2011.
- Baselt, R.C. Disposition of Toxic Drugs and Chemicals in Man, 2nd ed.; Biomedical Publications: Davis, CA, USA, 1982.
- Thurlkill, R.L.; Cross, D.A.; Scholtz, J.M.; Pace, C.N. Pka of fentanyl varies with temperature: Implications for acid-base management during extremes of body temperature. J. Cardiothorac. Vasc. Anesth. 2005, 19, 759–762, https://doi.org/10.1053/j.jvca.2004.11.039.
- Meuldermans, W.E.G.; Hurkmans, R.M.A.; Heykants, J.J.P. Plasma-protein binding and distribution of fentanyl, sufentanil, alfentanil and lofentanil in blood. Arch. Int. Pharmacodyn. Ther. 1982, 257, 4–19.
- Albrecht, J. Study of electrochemical transformations of new designer drugs. Master’s Thesis, Palacky University in Olomouc, Olomouc, Czech Republic, 2019.
- Misailidi, N.; Athanaselis, S.; Nikolaou, P.; Katselou, M.; Dotsikas, Y.; Spiliopoulou, C.; Papoutsis, I. A gc-ms method for the determination of furanylfentanyl and ocfentanil in whole blood with full validation. Forensic Toxicol. 2019, 37, 238–244, https://doi.org/10.1007/s11419-018-0449-2.
- Wilde, M.; Pichini, S.; Pacifici, R.; Tagliabracci, A.; Busardo, F.P.; Auwarter, V.; Solimini, R. Metabolic pathways and potencies of new fentanyl analogs. Front. Pharmacol. 2019, 10, 238, https://doi.org/10.3389/fphar.2019.00238.
- Vuckovic, S.; Prostran, M.; Ivanovic, M.; Dosen-Micovic, L.; Todorovic, Z.; Nesic, Z.; Stojanovic, R.; Divac, N.; Mikovic, Z. Fentanyl analogs: Structure–activity–relationship study. Curr. Med. Chem. 2009, 16, 2468–2474, https://doi.org/10.2174/092986709788682074.
- Bergh, M.S.S.; Bogen, I.L.; Nerem, E.; Wohlfarth, A.; Wilson, S.R.; Oiestad, A.M.L. Discovering the major metabolites of the three novel fentanyl analogues 3-methylcrotonylfentanyl, furanylbenzylfentanyl, and 4-fluorocyclopropylbenzylfentanyl for forensic case work. Forensic Toxicol. 2021, 39, 167–178, https://doi.org/10.1007/s11419-020-00560-9.
- DEA. Controlled Substances. Available online: https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf (accessed on 19 October 2020).
- Smith, H.S. Opioid metabolism. Mayo Clin. Proc. 2009, 84, 613–624.
- Labroo, R.B.; Paine, M.F.; Thummel, K.E.; Kharasch, E.D. Fentanyl metabolism by human hepatic and intestinal cytochrome p450 3a4: Implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab. Dispos. 1997, 25, 1072–1080.
- Armenian, P.; Vo, K.T.; Barr-Walker, J.; Lynch, K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 2018, 134, 121–132, https://doi.org/10.1016/j.neuropharm.2017.10.016.
- Burkle, H.; Dunbar, S.; VanAken, H. Remifentanil: A novel, short-acting, mu-opioid. Anesth. Analg. 1996, 83, 646–651, https://doi.org/10.1097/00000539-199609000-00038.
- Henkel, E.; Vella, R.; Behan, K.; Austin, D.; Kruger, P.; Fenning, A. The effect of concentration, reconstitution solution and pH on the stability of a remifentanil hydrochloride and propofol admixture for simultaneous co-infusion. BMC Anesthesiol. 2020, 20, 283, https://doi.org/10.1186/s12871-020-01194-5.
- Zawilska, J.B.; Kuczynska, K.; Kosmal, W.; Markiewicz, K.; Adamowicz, P. Carfentanil—From an animal anesthetic to a deadly illicit drug. Forensic Sci. Int. 2021, 320, 110715, https://doi.org/10.1016/j.forsciint.2021.110715.
- Feasel, M.G.; Wohlfarth, A.; Nilles, J.M.; Pang, S.K.; Kristovich, R.L.; Huestis, M.A. Metabolism of carfentanil, an ultrapotent opioid, in human liver microsomes and human hepatocytes by high-resolution mass spectrometry. AAPS J. 2016, 18, 1489–1499, https://doi.org/10.1208/s12248-016-9963-5.
- Sohouli, E.; Keihan, A.H.; Shahdost-fard, F.; Naghian, E.; Plonska-Brzezinska, M.E.; Rahimi-Nasrabadi, M.; Ahmadi, F. A glassy carbon electrode modified with carbon nanoonions for electrochemical determination of fentanyl. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110684, https://doi.org/10.1016/j.msec.2020.110684.
- Watanabe, S. Metabolic study of new psychoactive substances. Ph.D. Thesis, University of Technology, Sydney, Australia, 2018.
- Watanabe, S.; Vikingsson, S.; Roman, M.; Green, H.; Kronstrand, R.; Wohlfarth, A. In vitro and in vivo metabolite identifi-cation studies for the new synthetic opioids acetylfentanyl, acrylfentanyl, furanylfentanyl, and 4-fluoro-isobutyrylfentanyl. AAPS J. 2017, 19, 1102–1122, https://doi.org/10.1208/s12248-017-0070-z.
- Staeheli, S.N.; Baumgartner, M.R.; Gauthier, S.; Gascho, D.; Jarmer, J.; Kraemer, T.; Steuer, A.E. Time-dependent postmortem redistribution of butyrfentanyl and its metabolites in blood and alternative matrices in a case of butyrfentanyl intoxication. Forensic Sci. Int. 2016, 266, 170–177, https://doi.org/10.1016/j.forsciint.2016.05.034.
- Gampfer, T.M.; Wagmann, L.; Park, Y.M.; Cannaert, A.; Herrmann, J.; Fischmann, S.; Westphal, F.; Muller, R.; Stove, C.P.; Meyer, M.R. Toxicokinetics and toxicodynamics of the fentanyl homologs cyclopropanoyl-1-benzyl-4′-fluoro-4-anilinopiperidine and furanoyl-1-benzyl-4-anilinopiperidine. Arch. Toxicol. 2020, 94, 2009–2025, https://doi.org/10.1007/s00204-020-02726-1.
- Glasscott, M.W.; Vannoy, K.J.; Iresh Fernando, P.U.A.; Kosgei, G.K.; Moores, L.C.; Dick, J.E. Electrochemical sensors for the detection of fentanyl and its analogs: Foundations and recent advances. TrAC–Trends Anal. Chem. 2020, 132, 116037, https://doi.org/10.1016/j.trac.2020.116037.
- Shaabani, N.; Chan, N.W.C.; Jemere, A.B. A molecularly imprinted sol-gel electrochemical sensor for naloxone determination. Nanomaterials 2021, 11, 631, https://doi.org/10.3390/nano11030631.
- Marchei, E.; Pacifici, R.; Mannocchi, G.; Marinelli, E.; Busardo, F.P.; Pichini, S. New synthetic opioids in biological and non-biological matrices: A review of current analytical methods. TrAC Trends Anal. Chem. 2018, 102, 1–15, https://doi.org/10.1016/j.trac.2018.01.007.
- Lozier, M.J.; Boyd, M.; Stanley, C.; Ogilvie, L.; King, E.; Martin, C.; Lewis, L. Acetyl fentanyl, a novel fentanyl analog, causes 14 overdose deaths in Rhode Island, March–May 2013. J. Med. Toxicol. 2015, 11, 208–217, https://doi.org/10.1007/s13181-015-0477-9.
- Kumar, K.; Ballantyne, J.A.; Baker, A.B. A sensitive assay for the simultaneous measurement of alfentanil and fentanyl in plasma. J. Pharm. Biomed. Anal. 1996, 14, 667–673, https://doi.org/10.1016/0731-7085(95)01685-6.
- Gergov, M.; Nokua, P.; Vuori, E.; Qjanpera, I. Simultaneous screening and quantification of 25 opioid drugs in post-mortem blood and urine by liquid chromatography-tandem mass spectrometry. Forensic Sci. Int. 2009, 186, 36–43, https://doi.org/10.1016/j.forsciint.2009.01.013.
- Wang, L.Q.; Bernert, J.T. Analysis of 13 fentanils, including sufentanil and carfentanil, in human urine by liquid chromatography-atmospheric-pressure ionization-tandem mass spectrometry. J. Anal. Toxicol. 2006, 30, 335–341, https://doi.org/10.1093/jat/30.5.335.
- Kingsbury, D.P.; Makowski, G.S.; Stone, J.A. Quantitative-analysis of fentanyl in pharmaceutical preparations by gas-chromatography mass-spectrometry. J. Anal. Toxicol. 1995, 19, 27–30, https://doi.org/10.1093/jat/19.1.27.
- Wilson, N.G.; Raveendran, J.; Docoslis, A. Portable identification of fentanyl analogues in drugs using surface-enhanced Raman scattering. Sens. Actuat. B-Chem. 2021, 330, 129303, https://doi.org/10.1016/j.snb.2020.129303.
- Slepchenko, G.B.; Gindullina, T.M.; Nekhoroshev, S.V. Capabilities of the electrochemical methods in the determination of narcotic and psychotropic drugs in forensic chemistry materials. J. Anal. Chem. 2017, 72, 703–709, https://doi.org/10.1134/S1061934817070127.
- Lin, Y.; Sun, J.F.; Tang, M.; Zhang, G.H.; Yu, L.; Zhao, X.B.; Ai, R.; Yu, H.L.; Shao, B.; He, Y. Synergistic recognition-triggered charge transfer enables rapid visual colorimetric detection of fentanyl. Anal. Chem. 2021, 93, 6544–6550, https://doi.org/10.1021/acs.analchem.1c00723.
- Roda, G.; Faggiani, F.; Bolchi, C.; Pallavicini, M.; Dei Cas, M. Ten years of fentanyl-like drugs: A technical-analytical review. Anal. Sci. 2019, 35, 479–491, https://doi.org/10.2116/analsci.18R004.
- Alburges, M.E.; Hanson, G.R.; Gibb, J.W.; Sakashita, C.O.; Rollins, D.E. Fentanyl receptor assay 2. Utilization of a radioreceptor assay for the analysis of fentanyl analogs in urine. J. Anal. Toxicol. 1992, 16, 36–41, https://doi.org/10.1093/jat/16.1.36.
- Peng, L.J.; Wen, M.L.; Yao, Y. Potentiometric determination of fentanyl in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002, 30, 667–673, https://doi.org/10.1016/S0731-7085(02)00345-X.
- Garrido, J.M.P.J.; Delerue-Matos, C.; Borges, F.; Macedo, T.R.A.; Oliveira-Brett, A.M. Electrochemical analysis of opiates—An overview. Anal. Lett. 2004, 37, 831–844, https://doi.org/10.1081/Al-120030282.
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Recent biosensing advances in the rapid detection of illicit drugs. TrAC–Trends Anal. Chem. 2020, 131, 116006, https://doi.org/10.1016/j.trac.2020.116006.
This entry is offline, you can click here to edit this entry!
