Current therapies for high-grade gliomas, particularly glioblastomas (GBM), do not extend patient survival beyond 16–22 months. OKN-007 (OKlahoma Nitrone 007), which is currently in phase II (multi-institutional) clinical trials for GBM patients, and has demonstrated efficacy in several rodent and human xenograft glioma models, shows some promise as an anti-glioma therapeutic, as it affects most aspects of tumorigenesis (tumor cell proliferation, angiogenesis, migration, and apoptosis). Combined with the chemotherapeutic agent temozolomide (TMZ), OKN-007 is even more effective by affecting chemo-resistant tumor cells.
1. Introduction
Gliomas can either be astrocytomas, oligodendrogliomas, ependymomas, or mixed neuronal-glial tumors, with grades varying from I (least malignant) to IV (most malignant) as established by the World Health Organization (WHO) [
1]. High-grade gliomas (HGGs) consist of grade III and IV tumors. Unfortunately, patient survival for grade IV gliomas is between 16–22 months with current standard-of-care (SOC) treatments [
1], which includes surgical tumor resection, and follow up therapies including radiation, chemotherapy (temozolomide or TMZ (the most commonly used)) and possible treatment with bevacizumab (an antibody therapy targeting the vascular endothelial growth factor or VEGF).
Our group (Towner) has been studying the effect of a small molecular weight (MW), anti-inflammatory molecule, OKN-007 (disodium 4-[(tert-butyl-imino) methyl] benzene-1,3-disulfonate
N-oxide; or 2,4-disulfophenyl-
N-tert-butyl nitrone or, OKlahoma Nitrone 007), for the past decade as a therapeutic agent against HGGs, particularly grade IV glioblastomas (GBM). OKN-007 is currently in phase II multi-institutional clinical trials for both recurrent and newly diagnosed GBM patients in combination therapy with TMZ and radiation. In preclinical studies, our group found that OKN-007 was very effective in significantly reducing tumor volumes and elevating animal survival in various high-grade, orthotopic, glioma models, including rat C6 [
2], F98 [
3,
4], mouse GL261 [
5], and human adult U87 [
3] and G55 [
6], as well as pediatric patient-derived GBM [
7] and patient-derived diffuse intrinsic pontine glioma (DIPG) [
8] xenografts. In addition to effects on animal survival and tumor volumes, OKN-007 was found to significantly reduce necrosis in F98 rat gliomas [
9]. In the rat F98 glioma model, we also obtained RT-PCR and microarray data that indicated that OKN-007 acted through the transforming growth factor β (TGF-β1) pathway as a master regulator that down-regulated 57 genes connected with the extracellular matrix (ECM) [
6]. In the F98 glioma model, OKN-007 was found to decrease cell proliferation and angiogenesis and increase tumor cell apoptosis [
3,
9]. OKN-007 was also found to decrease the levels of in vivo free radicals [
4]. Additionally, we discovered that OKN-007 is able to augment a decrease in tumor cell growth when co-administered with TMZ in a human G55 xenograft model [
6].
The rat F98 high-grade glioma model has various characteristics that are closely associated with human HGGs, including an invasive tumor growth pattern and overexpression of RAS, PDGFB, EGFR, cyclin D1, and D2 [
10]. The F98 glioma cell line was initially generated via an intravenous (Iv) injection of ethyl nitrosourea (ENU) transplacentally into pregnant (20 days gestation) Fisher 344 rats, and then developed brain tumors following cloning when intracerebrally injected into syngeneic Fisher 344 rat brains [
10].
In this study, blood sera from non-tumor and F98 tumor-bearing rats were subjected to mass spectral (MS) analyses to determine if MS-MS-isolated proteins from these three treatment groups varied and could be used to elucidate distinctive protein profiles of interest that could help establish some further insights, or support our gene data, regarding the mechanism-of-action (MOA) of OKN-007 in high-grade gliomas.
2. Current Insights
OKN-007, formerly known as NXY-059, was found to have no adverse effects in human safety/toxicity studies [
21,
22], and we have not seen any adverse effects in any of our preclinical studies. We also have previously shown that OKN-007 is able to cross the blood-brain barrier (BBB) [
4], and in fact, temporarily opens up the BBB for a brief 1–2 h period (based on its pharmacokinetics) [
23]; however, the mechanism-of-action is currently unknown. It should also be noted that several of the preclinical models for HGGs have “leaky” blood–tumor barriers.
From our previous microarray data assessing genes that were down-regulated by OKN-007 in F98 tumors, compared to untreated tumors, there were 57 genes all associated with the master regulator TGF-β1 [
6]. Most of the associated genes were related to the ECM [
6]. There were also indications that the mTOR pathway was affected by OKN-007 [
6]. Of importance to the proteins discussed below in this study were ITGA1 (integrin alpha1), 2 and 4 (not 3, however), and ADAMTS2 (not 18, however). ITGA1 is a pre-malignant biomarker, which usually fosters therapy resistance and metastatic potential in pancreatic cancer [
24]. Notch 3 activation was found to increase the expression of ITGA1 in ovarian cancer cells [
25]. ITGA2 is highly expressed in several GBM cell lines [
26]. ITGA4 is a metastasis-associated gene [
27]. ADAMTS-2 is in the ADAMTS family, and is a procollagen N-proteinase [
28]. ADAMTS-2 was found to be overexpressed in gastric cancer fibroblast cells [
28]. In addition to the effect of OKN-007 on the ECM as a single agent, our group also established that when OKN-007 is linked with TMZ, it is even more effective in significantly increasing animal survival and decreasing tumor volumes in a G55 high-grade glioma xenograft model [
6]. In vitro data also indicated that a combination of OKN-007 with TMZ also resulted in decreasing the cell proliferation of TMZ-resistant human GBM cell lines [
6].
From this study, proteins of interest identified by tandem MS-MS that were decreased in sera from tumor-bearing rats treated with OKN-007, compared to untreated, included ABCA2, ATP5B, CNTN2, ITGA3, KMT2D, MYCBP2, NOTCH3, and VCAN. ABCA2 is part of the adenosine triphosphate-binding cassette transporter superfamily and is thought to exert important roles in the transmembrane transport of endogenous lipids, including myelin [
29]. The relative expression level of ABCA2 mRNA was found to be significantly higher in oligodendrogliomas compared to anaplastic astrocytomas or GBM [
29]. ATP5B has been found to be highly expressed in GBM tumor cells [
30]. CNTN2 was found to be highly expressed in oligodendrogliomas [
31]. ITGA3 is a cell surface adhesion protein that cooperates with ECM proteins which function in cancer metastasis [
32,
33]. This finding is somewhat supported by our previous gene data (see above). It was recently shown that brain-specific knockout of the H3K4 methyltransferase MLL4 (a COMPASS (COMplex of Proteins Associated with SET1)-like enzyme, also known as KMT2D) in mice spontaneously induces medulloblastoma [
34]. The MYC binding protein 2 (MYCBP2) was found to be a binding partner for the epidermal growth factor receptor (EGFR), which is frequently mutated in various cancers [
35]. It is well known that Notch3 activation promotes invasive glioma formation [
36]. Versican (VCAN) is a large chondroitin sulphate proteoglycan produced by many tumor cell types, which includes high-grade glioma [
37]. The increased expression of particular versican isoforms in the ECM is known to be involved in tumor cell growth, adhesion and migration [
37]. Transforming growth factor-beta2 (TGF-beta2) is an essential modulator of glioma invasion, in part via the remodeling of the ECM [
37]. Although our previous gene data indicated that TGF-β1 was the master regulator [
6], perhaps TGF-β2 also plays an integral role, which would have to be further studied in association with the MOA for OKN-007.
Conversely, proteins of interest in tumor-bearing rats elevated following OKN-007 treatment included ABCA6, ADAMTS18, VWA8, MACF1, and LAMA5. Glioma patients with elevated expression of ABCC8 mRNA were found to have a longer survival [
38]. ADAMTS18 is a part of the ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family proteins, which take part in vital roles in cancer progression and metastasis in various cancers [
39]. ADAMTS18 was previously found to be downregulated in numerous carcinoma cell lines, which suggested that it could be a tumor suppressor [
40]. von Willebrand factor A domain-containing 8 (VWA8) were found to be significantly downregulated in breast cancer brain metastases [
41]. Of some concern, MACF1 (microtubule actin cross-linking factor 1) was found to be predominately elevated in grade III-IV astrocytomas and grade IV glioblastoma, however when treated with TMZ, MACF1 is reduced and diminishes GBM cell proliferative capacity [
42]. Perhaps when we combine OKN-007 with TMZ [
6], the overall effect would be a decrease in MACF1, which we will have to study further in future studies. LAMA5 (laminin alpha5 subunit) is usually associated with cancer invasion/metastasis [
43], and we need to further assess the role of serum levels of detected LAMA5 in association with high-grade gliomas, which has not yet been investigated to our knowledge. Of note, in zebrafish xenografts for GBM (U251MG), it was shown that adhesion to LAMA5 was found to inhibit cell invasion [
44]. Our in vitro cell migration studies that assessed OKN-007 and OKN-007 combined with TMZ, indicated that G55 GBM cell migration was significantly decreased [
6]; however, we did not assess LAMA5 levels, which we would need to study in future studies. Another future study should involve assessing whether TMZ combined with OKN-007 could affect the levels of serum proteins further.
These findings, in general, support our previous gene analysis that indicates that OKN-007 may be effective against the ECM (see Figure 3). These findings also surmise that OKN-007 may be more effective against oligodendrogliomas and possibly other brain tumors such as medulloblastoma, as well as other types of cancers.
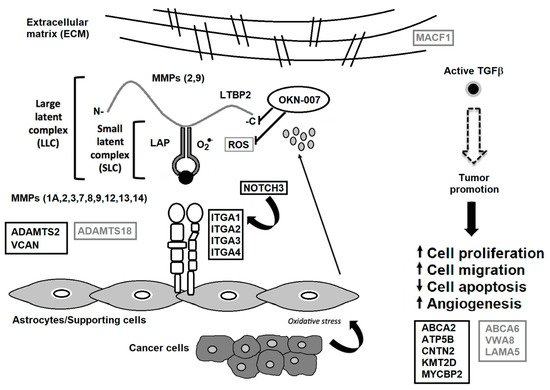
Figure 3. TGF-β associated with the tumor microenvironment. Some matrix metalloproteinases (MMPs) cleave LTBP, which releases latent TGF-β from the extracellular matrix (ECM). Various MMPs activate latent TGF-β through proteolytic cleavage of the latency-associated peptide (LAP), whereas integrins expressed on astrocytes (ITGA1, 2, 3 and 4) bind to the large latent complex (LLC), and activate latent TGF-β through MMP-dependent cleavage of LAP. Integrins (ITGA1, 2, 3 and 4) bind to the LLC and induce conformational changes in the latent complex through contractile action from activated astrocytes. Reactive oxygen species (ROS) produced by activated astrocytes from the induction of oxidative stress from nearby cancer cells may lead to the oxidation of the LAP domain and induce allosteric changes that release mature TGF-β from LAP. The mature (active) form of TGF-β can then bind to its receptor and then turn on tumor-promoting and tumor-suppressive properties. NOTCH3 activates ITGA1 [
25], which are both decreased by OKN-007. VCAN is highly expressed in high-grade gliomas [
37], which is decreased by OKN-007. ADAMTS2 is highly expressed in some cancers [
28], which is decreased by OKN-007. ADAMTS18 is a tumor suppressor [
40], which is elevated by OKN-007. During tumor promotion, activated TGF- β led to decreased apoptosis and increased cell proliferation, cell migration, and angiogenesis [
45]. OKN-007 is thought to act via LTBP and downregulates several genes associated with the ECM [
6], and is also a free radical scavenger [
4], resulting in the reversal of the major tumorigenic characteristics, i.e., increases tumor cell apoptosis and decreases cell proliferation, migration and vascular angiogenesis [
46]. Other proteins decreased by OKN-007 include ABCA2, ATP5B, CNTN2, KMT2D, and MYCBP2. Other proteins elevated by OKN-007 include ABCA6, VWA8, LAMA5, and MACF1. Serum proteins decreased by OKN-007 are highlighted in black rectangular boxes. Serum proteins elevated by OKN-007 are depicted in gray rectangular boxes. Previous down-regulated genes include
ITGA1, and
4,
ADAMTS2,
MMP 3, and
12, as well as several collagen genes (
COL1A1,
COL3A1,
COL4A1,
COL5A1,
COL6A2, and
COL7A1) [
6]. Modified from Towner et al. [
6] and Costanza et al. [
45].
This entry is adapted from the peer-reviewed paper 10.3390/brainsci12010100