Silver nanoparticles (AgNPs) have been implemented in a wide range of commercial products, resulting in their unregulated release into aquatic as well as terrestrial systems.Once released into the environment, they are prone to various transformation processes that modify their reactivity. In order to increase AgNP stability, different stabilizing coatings are applied during their synthesis. However, coating agents determine particle size and shape and influence their solubility, reactivity, and overall stability as well as their behavior and transformations in the biological medium. The employment of different stabilizing coatings can modulate AgNP-induced phytotoxicity with respect to growth, physiology, and gene and protein expression in terrestrial and aquatic plants and freshwater algae.
- silver nanoparticles
- plants
- green algae
- growth
- photosynthesis
1. Introduction
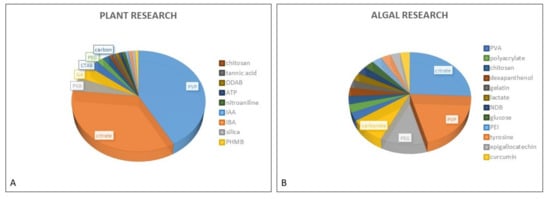 Figure 1. Proportional representation of coatings used for AgNP stabilization in plant (A) and algal (B) research.
Figure 1. Proportional representation of coatings used for AgNP stabilization in plant (A) and algal (B) research.2. AgNP Stability in Various Exposure Media
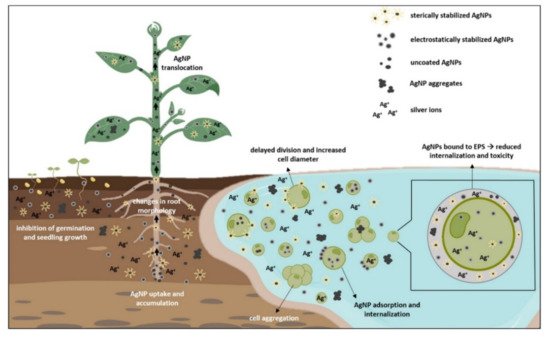
3. Silver Uptake and Effects on Growth and Morphology
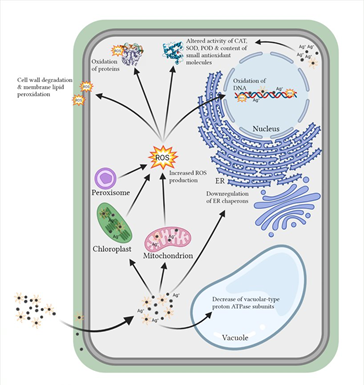
4. Oxidative Stress Induction and Mobilization of Antioxidant Machinery
Studies have shown that AgNPs are contributing to production of reactive oxygen species (ROS) [1][19], which induce oxidative stress in plant and algal cells by combined effects of direct surface oxidation and ability of ROS species to react with important biomolecules (Figure 3), which under severe oxidative stress can even lead to the cell death. Indirectly, release of Ag+ ions from AgNPs and properties of their coatings affect AgNP toxicity and additionally contribute to ROS production in promotion of oxidative stress.
Although oxidative stress plays an important role in the toxicity mechanism of AgNPs, it is still not clear whether production of ROS is a direct or indirect effect through release of Ag+ ions, as in most of the studies evaluated in this review detailed analysis of AgNPs stability is missing. Differentially coated AgNPs show different extents of ROS production as well as altered activity of antioxidant enzymes. The overall results suggest that coating-stabilized AgNPs influence plant and green algae response to stressful conditions in time- and concentration-dependent manner, although plant developmental stage can also interfere with the extent of AgNP-induced oxidative stress.
5. Impact on Photosynthesis
Most research to date shows that photosynthesis, the most important biochemical process on Earth for providing energy and oxygen, is particularly sensitive to AgNPs (Figure 4). Several studies have reported a decrease in chlorophyll and carotenoid content upon exposure to uncoated AgNPs in freshwater algae as well as vascular plants [1].
Although the exact mechanism of AgNP phytotoxicity is still not fully understood, AgNPs in the cell may dissociate to the toxic Ag+ ions [1][19]. They can competitively replace Cu+ ions in plastocyanin, an electron carrier in the photosynthetic electron-transfer chain, resulting in the disturbance of the photosynthetic electron transport and ROS generation. Furthermore, Ag+ can interact with the thiol group of enzymes of chlorophyll biosynthesis, thus interfering with this process. Another possible explanation for impaired photosynthesis could be diminished transpiration rate and stomatal conductance, resulting in lower rate of gas exchange and reduced CO2 assimilation.
Exposure of plants and freshwater algae to AgNPs with different surface coatings can cause both structural changes of the photosynthetic apparatus and functional ones that manifest as a decrease in the content of photosynthetic pigments, as well as an inhibition of photochemical reactions and CO2 assimilation. The divergence of the AgNP-induced effects on photosynthesis can be partly attributed to differences in physicochemical characteristics of AgNPs and their bioavailability imposed by different surface coatings. However, the effect of other factors such as plant species, developmental phase, type, and time of exposure should be also considered.
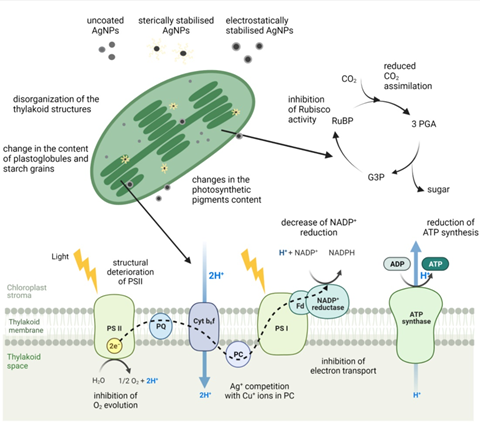
Figure 4. Structural and functional changes of the photosynthetic apparatus in plants and freshwater algae upon exposure to AgNPs with different surface coatings. RuBP - ribulose 1,5-bisphosphate, 3-PGA - 3-phosphoglyceric acid, G3P -glyceraldehyde 3-phosphate, PS - photosystem, PQ - plastoquinone, Cyt b6f - cytochrome b6f, PC - plastocyanin, Fd - ferredoxins. Figure was adapted from “Light Dependent Reactions of Photosynthesis” by BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates. Accessed November 24th, 2021.
6. Changes in Gene and Protein Expression
The application of AgNPs modulates morphophysiological, biochemical, and molecular status of plants and freshwater green algae [1][19]. In spite of the attention that nanomaterial phytotoxicity attracted in recent years, only limited investigations have been conducted on the molecular level effects of AgNPs in plants, while for studies on green algae, even less information in the literature can be found. To examine the molecular bases of AgNP phytotoxicity, gene and protein expression analyses have been performed in model as well as in different crop plants and only a few species of freshwater green algae.
Studies have shown that differently coated AgNPs have impact on gene and protein expression in various plant and algae species. Information obtained from these studies increase our understanding of the mechanisms involved in plant and green algae responses to AgNPs, which is relevant for environmental assessments. However, it is difficult to draw unambiguous conclusions since these studies have been investigated in different species, applied different concentrations of AgNPs with different coatings, and employed different exposure times. Therefore, in order to investigate the role of stabilizing coatings in AgNP-induced phytotoxicity on molecular level and to be able to compare different coatings, it would be useful to conduct a research that implemented differently coated AgNPs in the same experimental setup.
7. Conclusion
AgNP behavior in plant and algal exposure systems is dependent on surface coatings. On one hand, they stabilize nanoparticles, but on the other hand, are responsible for their physiochemical modifications, such as changes in aggregation and agglomeration, oxidation states, and dissolution rate of Ag+ ions. Surface coating-determined AgNP properties play an important role in AgNP uptake and modulate their effects on germination and development in plants. In algae, EPS plays an important role in AgNP bioaccumulation, which is why effects of differently coated AgNPs on EPS should be further investigated. Oxidative stress is proved to be the one of the major mechanisms of the AgNP-induced phytotoxicity in both plants and algae, although application of certain surface coatings seems to alleviate AgNP-induced ROS formation. The process of photosynthesis, in all its complexity, has been particularly affected by AgNPs, although algae, being unicellular organisms, seem to be more susceptible compared to plants. At the molecular level, gene and protein expression analyses confirmed AgNP-generated induction of oxidative stress and photosynthesis as the most sensitive target of AgNP toxic action, regardless of which coating is applied. However, in order to investigate the role of stabilizing coatings in AgNP-induced phytotoxicity on molecular level and to be able to compare different coatings, it would be useful to conduct a more studies which will implement differently coated AgNPs in the same experimental setup.
This entry is adapted from the peer-reviewed paper 10.3390/nano12010024
References
- Tkalec, M.; Peharec Štefanić, P.; Balen, B. Phytotoxicity of silver nanoparticles and defence mechanisms. Compr. Anal. Chem. 2019, 84, 145–198.
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562.
- Ray, Paresh Chandra; Hongtao, Y.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. Env. Sci Heal. C Env. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35.
- Smita, S.; Gupta, S.K.; Bartonova, A.; Dusinska, M.; Gutleb, A.C.; Rahman, Q. Nanoparticles in the environment: Assessment using the causal diagram approach. Environ. Heal. A Glob. Access Sci. Source 2012, 11, S13.
- Bae, S.; Hwang, Y.S.; Lee, Y.-J.; Lee, S.-K. Effects of water chemistry on aggregation and soil adsorption of silver nanoparticles. Environ. Health Toxicol. 2013, 28, e2013006.
- Behra, R.; Sigg, L.; Clift, M.J.D.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: From a chemical and biochemical perspective. J. R. Soc. Interface 2013, 10.
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914.
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalt. Trans. 2015, 44, 17883–17905.
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012.
- Zhao, C.M.; Wang, W.X. Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 2012, 6, 361–370.
- Liu, W.; Zhou, Q.F.; Liu, J.Y.; Fu, J.J.; Liu, S.J.; Jiang, G. Bin Environmental and biological influences on the stability of silver nanoparticles. Chinese Sci. Bull. 2011, 56, 2009–2015.
- Argentiere, S.; Cella, C.; Cesaria, M.; Milani, P.; Lenardi, C. Silver nanoparticles in complex biological media: Assessment of colloidal stability and protein corona formation. J. Nanoparticle Res. 2016, 18.
- Pem, B.; Ćurlin, M.; Jurašin, D.D.; Vrček, V.; Barbir, R.; Micek, V.; Fratila, R.M.; de la Fuente, J.M.; Vrček, I.V. Fate and transformation of silver nanoparticles in different biological conditions. Beilstein J. Nanotechnol. 2021, 12, 665–679.
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16.
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 1–16.
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017.
- Azodi, M.; Sultan, Y.; Ghoshal, S. Dissolution behavior of silver nanoparticles and formation of secondary silver nanoparticles in municipal wastewater by single-particle ICP-MS. Environ. Sci. Technol. 2016, 50, 13318–13327.
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350.
- Biba, R.; Peharec Štefanić, P.; Cvjetko, P.; Tkalec, M.; Balen, B. Silver nanoparticles phytotoxicity mechanisms. In Nanobiotechnology for Plant Protection; Silver Nanomaterials for Agri-Food Applications; Kamel, A.A.-E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 317–356. ISBN 9780128235287.
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34.
- Muraleetharan, V.; Mantaj, J.; Swedrowska, M.; Vllasaliu, D. Nanoparticle modification in biological media: Implications for oral nanomedicines. RSC Adv. 2019, 9, 40487–40497.
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414.
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834.
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857.
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59.
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305.
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745.
- Tymoszuk, A. Silver nanoparticles effects on in vitro germination, growth, and biochemical activity of tomato, radish, and kale seedlings. Materials 2021, 14.
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964.
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531.
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061.
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003.
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture 2020, 10, 312.
