Astaxanthin is a member of the carotenoid family that is found abundantly in marine organisms. It has been reported that astaxanthin functions both as a pigment, and as an antioxidant with superior free radical quenching capacity.
- astaxanthin
- obesity
- mitochondria
- energy metabolisms
- natural antioxidant
- insulin resistance
- AMPK
1. Introduction
1.1. Hidden Bioactivity of Natural Pigments
1.1.1. Nature Is Full of Splendid Color!
1.1.2. Carotenoids
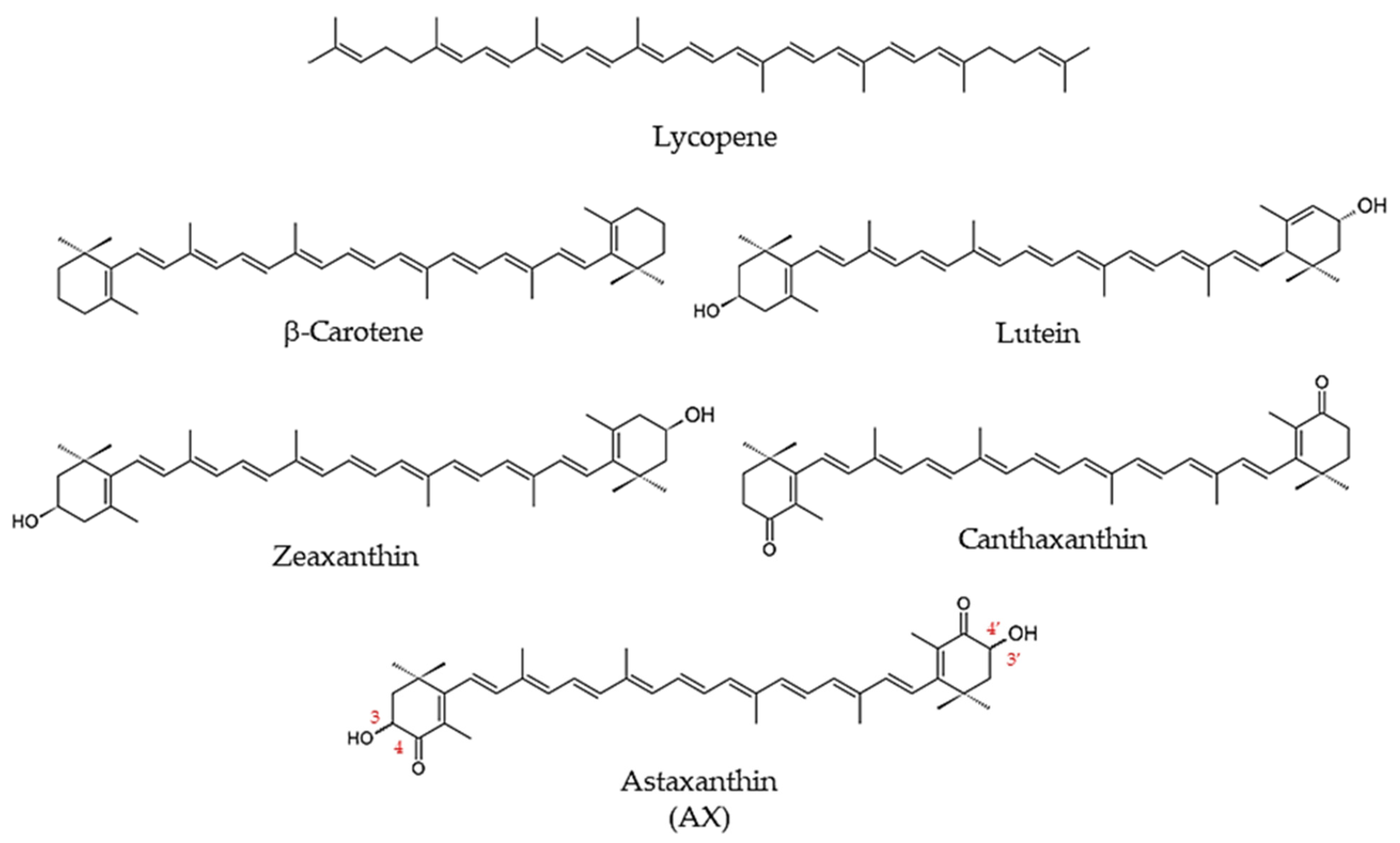
1.1.3. What Is Astaxanthin?
1.2. Biological Activity of Astaxanthin
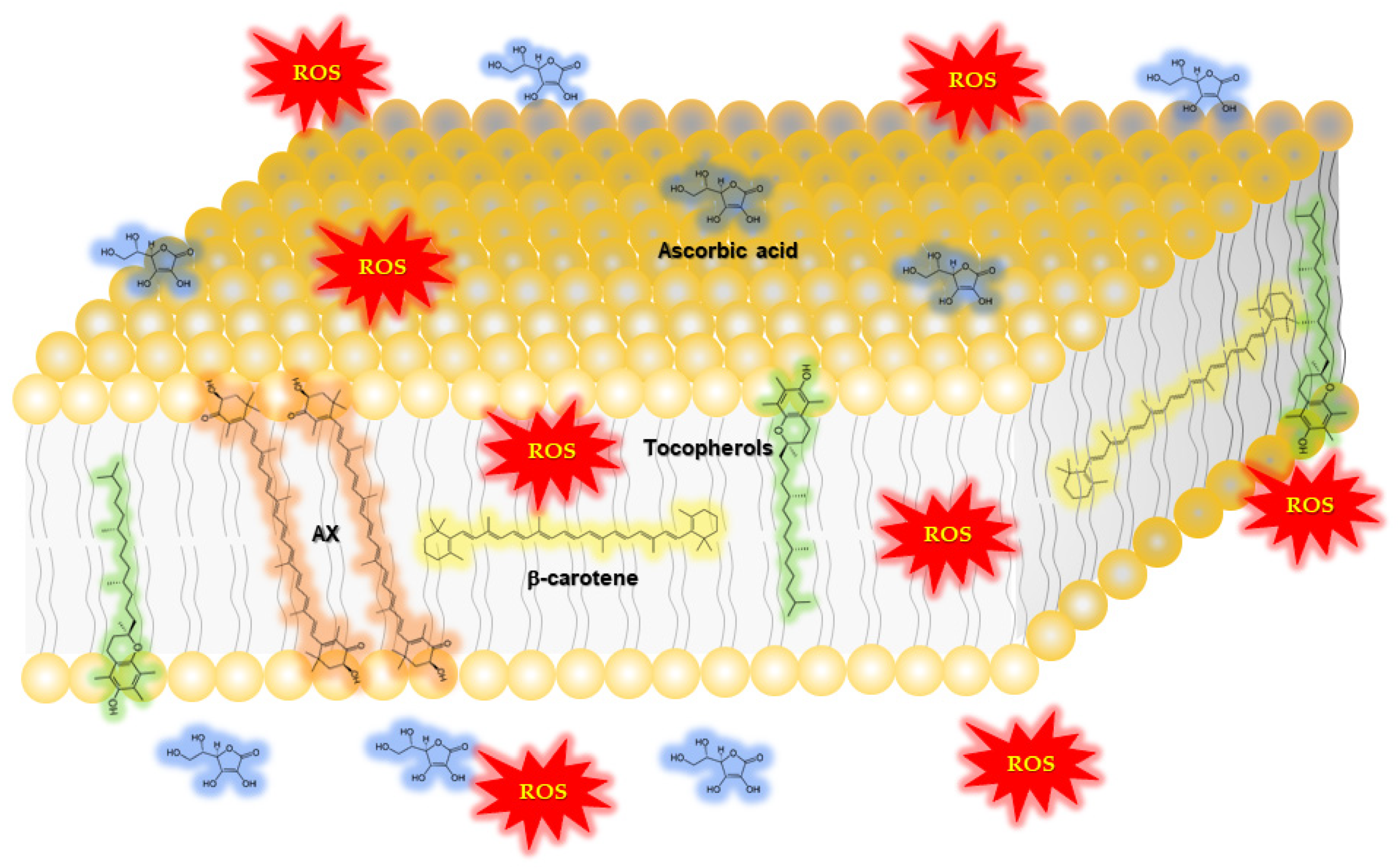
1.2.1. Function of Astaxanthin in Lipid Bilayers: Antioxidant Activity and Impact on Physical Properties
|
Author/Year/Reference |
Study Design |
Subjects |
Dose |
Duration |
Outcome |
|---|---|---|---|---|---|
|
McAllister M.J. et al., 2021 [30] |
Randomized, double-blind, placebo-controlled, crossover study |
14 healthy subjects |
0, 6 mg/day |
4 weeks |
Glutathione was ∼7% higher following AX compared with placebo (p < 0.05). No effect on plasma hydrogen peroxide or malondialdehyde (MDA; p > 0.05). Advanced oxidation protein products (AOPP) reduced by ∼28% (N.S.; p = 0.45). |
|
Petyaev I.M., et al., 2018 [31] |
Randomized, blinded, four-arm, prospective study |
32 subjects with oxidative stress, 8 subjects taking AX only |
0, 7 mg/day * |
4 weeks |
Reduced serum oxidized LDL by 55.4% after 4 weeks (p < 0.05). Reduced MDA by 52.7% after 4 weeks (p < 0.05). |
|
Chalyk, N. et al., 2017 [32] |
Open-label, prospective study |
31 subjects; 18 obese, 8 overweight, 5 healthy weight |
4 mg/day |
92 days |
Plasma MDA decreased with AX by 11.2% on day 15 and by 21.7% on day 29 (N.S.) |
|
Hashimoto H. et al., 2016 [33] |
Open-label, prospective study |
35 subjects during cataract surgery |
6 mg/day |
2 weeks |
Superoxide anion scavenging activity (U/mL) 18.2 ± 4.1 at 0 weeks reduced to 19.9 ± 3.6 after 2 weeks of supplementation compared with baseline, p < 0.05. Total hydroperoxides (U CARR) from 1.16 ± 0.18 at 0 weeks reduced to 1.04 ± 0.31 after 2 weeks of supplementation compared with baseline, p < 0.05 |
|
Baralic, I. et al., 2015 [34] |
Randomized, double-blind, placebo-controlled, prospective study |
40 healthy subjects (soccer players) |
0, 4 mg/day |
90 days |
Improved prooxidant-antioxidant balance (PAB; p < 0.05) |
|
Baralic I. et al., 2013 [35] |
Randomized, double-blind, prospective study |
40 healthy subjects (soccer players) |
0, 4 mg/day |
90 days |
Protected thiol groups against oxidative modification (increase in -SH groups, p < 0.05; improved PON1 activity towards paraoxon and diazoxon, p < 0.05 and p < 0.01, respectively) |
|
Hashimoto, H. et al., 2013 [36] |
Open-label, prospective study |
35 cataract patients |
6 mg/day |
2 weeks |
Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05), increased superoxide scavenging activity (p< 0.05) |
|
Choi H.D. et al., 2011 [37] |
Randomized, two-arm, prospective study |
23 obese and overweight subjects |
5 and 20 mg/day |
3 weeks |
5 mg/day: MDA decreased by 34.6%, isoprostane (ISP) decreased by 64.9%, superoxide dismutase (SOD) increased by 193%, and total antioxidant capacity (TAC) increased by 121% after 3 weeks compared with baseline (p < 0.01). 20 mg/day: MDA decreased by 35.2%, ISP decreased by 64.7%, SOD increased by 194%, and TAC increased by 125% after 3 weeks compared with baseline (p < 0.01). |
|
Choi, H.D. et al., 2011 [38] |
Randomized, double-blind, placebo-controlled, prospective study |
27 overweight subjects |
0, 20 mg/day |
12 weeks |
MDA reduced by 17.3% and 29% after 8 and 12 weeks compared with placebo (p < 0.01), isoprostane (ISP) reduced by 40.2% and 52.9% after 8 and 12 weeks compared with placebo (p < 0.01), superoxide dismutase (SOD) increased by 124.8% after 12 weeks compared with placebo (p < 0.01), and total antioxidant capacity (TAC) increased by 130.1% after 12 weeks compared with placebo (p < 0.05) (See Table 3 for other outcomes.) |
|
Hashimoto H. et al., 2011 [39] |
Open-label, prospective study |
35 cataract patients |
6 mg/day |
2 weeks |
Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) |
|
Kim, J.H. et al., 2011 [40] |
Randomized, Repeated, measured, prospective study |
39 heavy smokers, 39 non-smokers |
0, 5, 20, or 40 mg/day |
3 weeks |
5 mg/day: MDA and ISP significantly lower after 2 and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05) 20 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). 40 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 2 and 3 weeks compared with baseline in smokers (p < 0.05) |
|
Nakagawa K. et al., 2011 [41] |
Randomized, double-blind, placebo-controlled, prospective study |
30 healthy subjects |
0, 6, 12 mg/day |
12 weeks |
6 mg/day: reduction in total phospholipid hydroperoxides (PLOOH) after 12 weeks compared with baseline (p < 0.01) and compared with placebo (p < 0.05). Reduced phosphatidyl-ethanolamine hydroperoxide (PEOOH) after 12 weeks compared with baseline (p < 0.05) and compared with placebo (p < 0.05). 12 mg/day: 48% reduction in total PLOOH after 12 weeks compared with baseline (p < 0.01) and 35% less total PLOOH at 12 weeks compared with the control group (p < 0.05). The 12 mg/day group had 46% less phosphatidylcholine hydroperoxide (PCOOH) at 12 weeks compared with baseline (p < 0.01). |
|
Peng L. et al., 2011 [42] |
Randomized, placebo-controlled study |
115 healthy subjects |
0, 40 mg/day |
90 days |
Comparing with the control group, MDA contents in the test group decreased significantly (p < 0.01), and SOD and GSH-Px activities increased significantly (p < 0.01). |
|
Park J.S. et al., 2010 [43] |
Randomized, double-blind, placebo-controlled, prospective study |
42 healthy subjects |
2 or 8 mg/day |
8 weeks |
2 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05). 8 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05) |
|
Iwabayashi M. et al., 2009 [44] |
Open-label, prospective study |
35 healthy subjects (with high oxidative stress) |
12 mg/day |
8 weeks |
Increased blood biological antioxidant potential (BAP; +4.6%, p < 0.05) |
|
Yamada T. et al., 2010 [45] |
Open-label,prospective study |
6 healthy subjects and 6 Sjoegren’s syndrome subjects |
12 mg/day |
2 weeks |
Reduced protein oxidation (−10%, p < 0.05) |
|
Fassett, R.G. et al., 2008 [46] |
Randomized, double-blind, placebo-controlled, prospective study |
58 renal transplant recipients |
0, 12 mg/day |
12 months |
Total plasma F2-isoprostanes reduced by 23.0% in placebo and 29.7% in AX groups (N.S.) |
|
Karppi, J. et al., 2007 [47] |
Randomized, double-blind, placebo-controlled, prospective study |
39 healthy subjects |
0, 8 mg/day |
3 months |
Decreased oxidation of fatty acids in healthy men (p < 0.05) |
|
Kim Y.K. et al., 2004 [48] |
Open-label, prospective study |
15 healthy postmenopausal women |
0, 2, 8 mg/day |
8 weeks |
Decreased plasma TBARS levels: 2 mg group from 1.42 ± 0.18 to 1.13 ± 0.18 nM/mg (p < 0.05). 8 mg AX group from 1.62 ± 0.14 nM/mg to 1.13 ± 0.12 nM/mg after 8 weeks (p < 0.05). Increased TAS from 0.85 ± 0.42 mM/L to 1.90 ± 0.58 mM/L in the 8 mg group. Urinary 8-isoprostanes excretion did not decrease significantly. (See Table 3 for other outcomes.) |
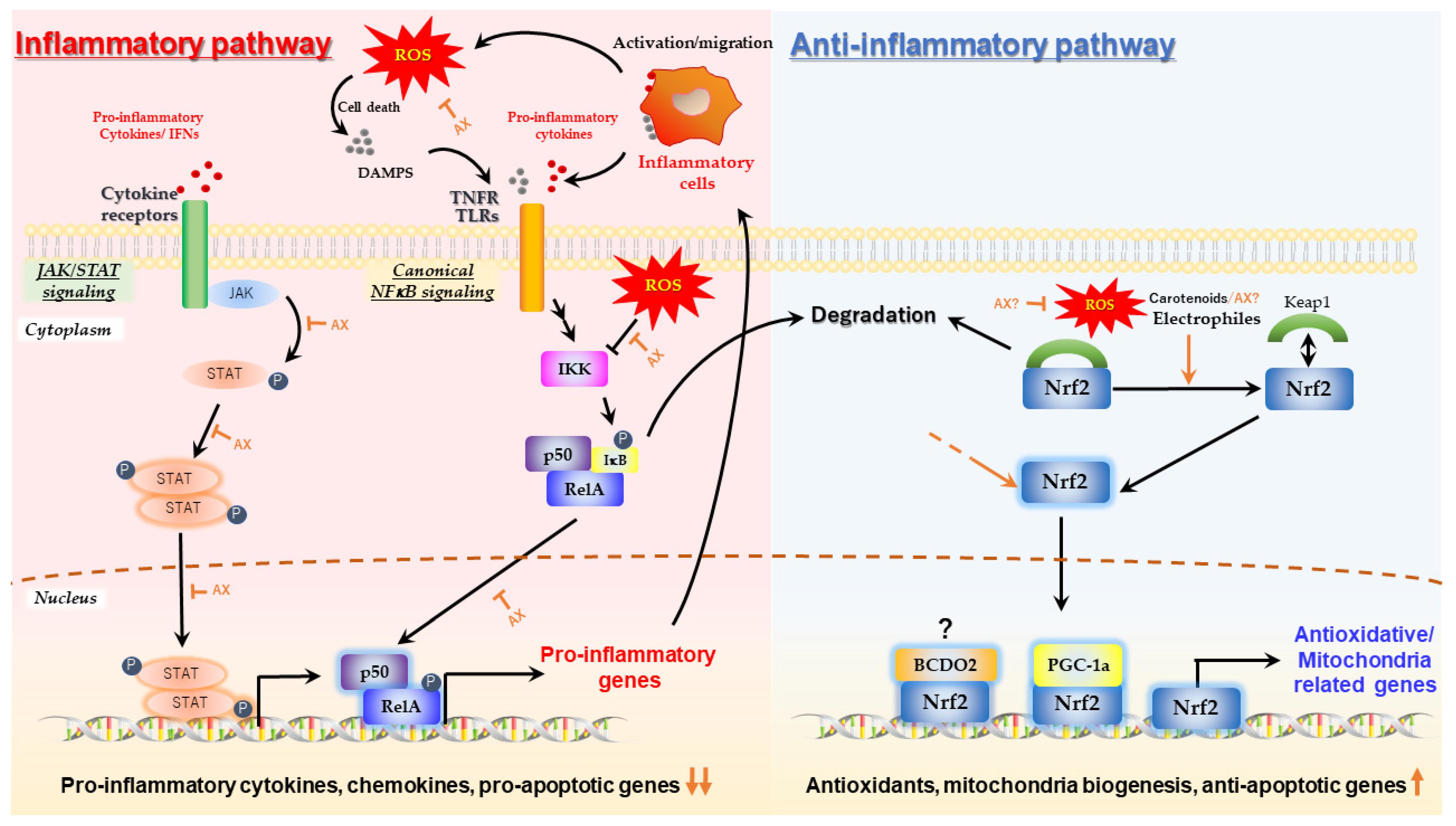
2. Mechanism by Which Astaxanthin Enhances Mitochondrial Energy Metabolism
2.1. Protective Effect of Astaxanthin on Mitochondria; Astaxanthin as a Mitochondrial Antioxidant
2.2. Aggressive Enhancement of Mitochondrial Activity and Metabolism via Gene Expression by Astaxanthin
3. Prospect of Astaxanthin for Human Health Promotion
|
Author/Year/Reference |
Study Design |
Subjects |
Dose |
Duration |
Outcome |
|---|---|---|---|---|---|
|
<Subjects: healthy athletes, high daily physical activity> |
|||||
|
Brown, R.D. et al., 2021 [100] |
Randomized, double-blind, placebo-controlled, crossover study |
12 recreationally trained male cyclists 27.5 ± 5.7 years, VO2peak: 56.5 ± 5.5 mL⋅kg−1⋅min−1, Wmax: 346.8 ± 38.4 W |
0, 12 mg/day |
7 days |
Completion time of the 40-km cycling time trial improved by 1.2 ± 1.7% with AX supplementation, from 70.76 ± 3.93 min in the placebo condition to 69.90 ± 3.78 min in the AX condition (mean improvement time = 51 ± 71 s, p = 0.029, g = 0.21). Whole body fat oxidation rate was also greater in the AX group between 39–40 km (+0.09 ± 0.13 g⋅min−1, p = 0.044, g = 0.52) and respiratory exchange ratio was lower (−0.03 ± 0.04, p = 0.024, g = 0.60). |
|
Talbott I. et al., 2018 [101] |
Randomized, double-blind, placebo-controlled, prospective study |
28 recreational runners (42 ± 8 years) |
0, 12 mg/day |
8 weeks |
Reduced average heart rate at submaximal endurance intensities (aerobic threshold, AeT and anaerobic threshold, AT), but not at higher “peak” intensities. |
|
Klinkenberg L.J. et al., 2013 [102] |
Randomized, double-blind, placebo-controlled prospective study |
32 well-trained male cyclists 25 ± 5 years, V˙O2peak = 60 ± 5 mL·kg−1·min−1, Wmax = 5.4 ± 0.5 W·kg−1 |
0, 20 mg/day * |
4 weeks |
N.S; effect on exercise-induced cardiac troponin T release (p = 0.24), changes in antioxidant capacity markers (trolox equivalent antioxidant capacity, uric acid, and malondialdehyde). Markers of inflammation (high-sensitivity C-reactive protein) and exercise-induced skeletal muscle damage (creatine kinase). |
|
Res T. et al., 2013 [103] |
Randomized, double-blind, placebo-controlled, prospective study |
32 trained male cyclists or triathletes 25 ± 1 years, V˙O2peak = 60 ± 1 mL·kg−1·min−1, Wmax = 395 ± 7 W |
0, 20 mg/day |
4 weeks |
N.S; total plasma antioxidant capacity (p = 0.90) or attenuated malondialdehyde levels (p = 0.63). Whole-body fat oxidation rates during submaximal exercise (from 0.71 +/− 0.04 to 0.68 ± 0.03 g⋅min−1 and from 0.66 ± 0.04 to 0.61 ± 0.05 g⋅min−1 in the placebo and AX groups, respectively; p = 0.73), time trial performance (from 236 ± 9 to 239 ± 7 and from 238 ± 6 to 244 ± 6 W in the placebo and AX groups, respectively; p = 0.63). |
|
Djordjevic B. et al., 2011 [104] |
Randomized, Double-blind, placebo-controlled, prospective study |
32 male elite soccer players |
0, 4 mg/day |
90 days |
Changes in elevated O2-¯ concentrations after soccer exercise were statistically significant only in the placebo group (exercise × supplementation effect, p < 0.05); TAS values decreased significantly only in the placebo group after exercise (p < 0.01). After intervention, total SH group content increased (21% and 9%, respectively), and the effect of AX was marginally significant (p = 0.08). Basal SOD activity was significantly reduced in both the placebo and AX groups at the end of the study (main training effect, p < 0.01). Post-exercise CK and AST levels were significantly lower in the AX group than in the placebo group (p < 0.05) |
|
Earnest C.P. et al., 2011 [105] |
Randomized, double-blind, placebo-controlled, prospective study |
14 amateur endurance-trained subjects 18–39 years, V˙O2peak = 52.84 ± 3.5 mL·kg−1·min−1, Wmax = 330 ± 26 W |
0, 4 mg/day |
28 days |
Improved performance in the 20-km cycling time trial in the AX group (n = 7, −121 s; 95% CI, −185, −53), but not in the placebo group (n = 7, −19 s; 95% CI, −84, 45). AX group significantly increased power output (20 W; 95% CI, 1, 38), whereas the placebo group did not (1.6 W; 95% CI, −17, 20). N.S; carbohydrate, fat oxidation and blood indices indicative of fuel mobilization. |
|
Bloomer, R.J. et al., 2005 [106] |
Randomized, placebo-controlled, prospective study |
20 resistance trained male subjects (25.1 ± 1.6 years) |
0, 4 mg/day * |
3 months |
N.S; Muscle soreness, creatine kinase (CK), and muscle performance were measured before and through 96-h post-eccentric exercise |
|
Sawaki K. et al., 2002 [107] |
Randomized double-blind placebo-controlled, prospective study |
16 healthy adult male subjects |
0, 6 mg/day |
4 weeks |
In the AX group, the serum lactate concentration after 2 min of activity (1200 m run) was significantly lower than that in the control group. |
|
<Subjects: healthy subjects> |
|||||
|
Kawamura A. et al., 2021 [108] |
Randomized controlled open-label, prospective study |
26 healthy male subjects |
N/A (1 mg AX/100 g salmon) * |
10 weeks |
The skeletal muscle mass was higher after training than before training in both control and intervention groups (p < 0.05). Increased maximal voluntary contraction after training in the intervention group (p < 0.05), but not significantly increased in the control group. (See Table 3 for other outcomes.) |
|
Fleischmann C. et al., 2019 [109] |
Randomized, double-blind, placebo-controlled, prospective study |
22 healthy subjects |
0, 12 mg/day |
30 days |
Decreased raise in blood lactate caused by the VO2 Max test; AX group (9.4 ± 3.1 and 13.0 ± 3.1 mmole⋅L−1 in the AX and placebo groups, respectively p < 0.02). Change in oxygen uptake during recovery (−2.02 ± 0.64 and 0.83 ± 0.79% of VO2 Max in the AX and placebo group, respectively, p = 0.001). N.S; anaerobic threshold or VO2 Max. physiological or biochemical differences in the heat tolerance test (HTT) (2 h walk at 40 °C, 40% relative humidity. |
|
Takami M. et al., 2019 [110] |
Open-label, prospective study |
20 healthy young male subjects |
c.a, 4.5 mg/day * from salmon |
4 weeks |
Increased maximum workload by training in both groups (p = 0.009), and increased oxygen consumption during exercise in the antioxidant group only (p = 0.014). There were positive correlations between maximum workload and fat (r = 0.575, p = 0.042) and carbohydrate oxidations (r = 0.520, p = 0.059) in the antioxidant group. (See Table 3 for other outcomes.) |
|
Imai A. et al., 2018 [111] |
Randomized, double-blind, placebo-controlled, crossover study |
42 healthy subjects |
0, 6 mg/day * |
4 weeks |
Elevated PCOOH levels during mental and physical tasks were attenuated by AX supplementation. Improved recovery from mental fatigue compared with the placebo. No differences were found between AX and the placebo in other secondary outcomes, such as subjective feelings, work efficiency, and autonomic activity. |
|
Hongo N. et al., 2017 [112] |
Randomized, double-blind placebo-controlled, prospective study |
39 healthy subjects |
0, 12 mg/day * |
12 weeks |
Intent-to-treat (ITT) analysis; fatigue after physical and mental stress was significantly lower in the AX group than in the placebo at week 8; the change in POMS Friendliness was significantly higher in the AX group than in the control group at week 8; the rate of change in BAP values at week 12 was not significantly different between the AX and control groups. The rate of change in BAP values at week 12 was not significantly different between the AX group and the control. |
|
Malmstena C.L.L. et al., 2008 [113] |
Randomized, double-blind, placebo-controlled, prospective study |
40 young healthy subjects (17–19 years) |
0, 4 mg/day |
3 months |
Increased average number of knee bending (squats) increased by 27.05 (from 49.32 to 76.37, AX group) vs. 9.0 (from 46.06 to 55.06, placebo subjects), p = 0.047. |
|
Tajima T. et al., 2004 [114] |
Randomized, double-blind, placebo-controlled, crossover study |
18 healthy subjects (35.7 ± 4 years) |
0, 5 mg/day |
2 weeks |
Increased in CVRR and HF/TF (Heart rate variability) were significant during exercise at 70% maximum heart rate (HRmax) intensity (p < 0.05). Also, after the AX supplementation, decreased minute ventilation (VE) during exercise at 70% HRmax (p < 0.05). Decreased LDL cholesterol (chol) (p < 0.05) and respiratory quotient after exercise. |
|
<Subjects: elderly subjects> |
|||||
|
Liu S.Z. et al., 2021 [115] |
Randomized, double-blind, placebo-controlled, prospective study |
42 elderly subjects (65–82 years) |
0, 12 mg/day * |
12 weeks |
In endurance training (ET), specific muscular endurance was improved only in the AX group (Pre 353 ± 26 vs. Post 472 ± 41) and submaximal graded exercise test duration was improved in both groups (placebo 40.8 ± 9.1% vs. AX 41.1 ± 6.3%). The increase in fat oxidation at low intensity after ET was greater in AX (placebo 0. 23 ± 0.15 g vs. AX 0.76 ± 0.18 g), and was associated with reduced carbohydrate oxidation and improved exercise efficiency in men, but not in women. |
|
Liu S.Z. et al., 2018 [116] |
Randomized double-blind, placebo-controlled, prospective study |
42 elderly subjects (65–82 years) |
0, 12 mg/day * |
12 weeks |
Administration of AX increased maximal voluntary force (MVC) by 14.4% (± 6.2%, p < 0.02), tibialis anterior muscle size (cross-sectional area, CSA) by 2.7% (± 1.0%, p < 0.01), and specific impulse increased by 11.6% (MVC/CSA, ± 6.0%, p = 0.05), respectively, whereas placebo treatment did not alter these characteristics (MVC, 2.9% ± 5.6%; CSA, 0.6% ± 1.2%; MVC/CSA, 2.4 ± 5.7%; all p > 0.6). |
|
Fujino H. et al., 2016 [117] |
Randomized, double-blind, placebo-controlled, prospective study |
29 community-dwelling healthy elderly subjects (80.9 ± 1.5 years.) |
0, 12 mg/twice a day * |
3 months |
Decrease in d-ROM values with AX group (p < 0.01), but not the placebo group; the AX group had a therapeutic effect on 6-min walking distance compared with the placebo group (p < 0.05). AX group had an increase in distance and number of steps in the 6-min walking test compared with the placebo group. Furthermore, the rate of increase in blood lactate levels after walking was lower in the AX group than in the placebo group (p < 0.01). |
|
Author/Year/Reference |
Study Design |
Subjects |
Dose |
Duration |
Outcome |
|---|---|---|---|---|---|
|
Shokri-Mashhadi, N. et al., 2021 [118] |
Randomized, double-blind, placebo-controlled, prospective study |
44 patients with type 2 diabetes |
0, 8 mg/day |
8 weeks |
Decrease plasma levels of MDA and IL-6 (p < 0.05) and decrease the expression level of miR-146a, associated with inflammatory markers (fold change: −1/388) (p < 0.05). |
|
Kawamura A. et al., 2021 [108] |
Randomized controlled Open-label, prospective study |
26 healthy male subjects |
N/A (1 mg AX/100 g salmon) * |
10 weeks |
Higher resting oxygen consumption after training in the intervention group only (p < 0.05). Serum carbonylated protein level as an oxidative stress marker tended to be lower immediately after exercise than before exercise in the intervention group only (p = 0.056). (See Table 2. for other outcomes.) |
|
Kato T. et al., 2020 [119] |
Open-label, prospective study |
16 patients with systolic heart failure |
12 mg/day * |
3 months |
Increased left ventricular ejection fraction (LVEF) from 34.1 ± 8.6% to 38.0 ± 10.0% (p = 0.031) and 6-min walk distance increased from 393.4 ± 95.9 m to 432.8 ± 93.3 m (p = 0.023). Significant relationships were observed between percent changes in dROM level and those in LVEF. |
|
Chan K. et al., 2019 [120] |
Randomized controlled Open-label, prospective study |
54 patients with type 2 diabetes |
0, 6, 12 mg/day |
8 weeks |
Increased plasma AX levels and decreased fasting plasma glucose and HbA1c levels. In 12 mg AX group, reduced in plasma triglyceride, total chol and LDL levels. Lowered changes in plasma IL-6 and TNF-α levels and plasma vWF level and higher changes in AT-III level. In 12 mg AX group, decreased changes in plasma FVII and PAI-1 levels. |
|
Takami M. et al., 2019 [121] |
Open-label, prospective study |
20 healthy young male subjects |
c.a, 4.5 mg/day * from salmon |
4 weeks |
Higher carbohydrate oxidation during rest in the post-training than that in the pre-training only in the antioxidant group. More decreased levels of serum insulin and HOMA-IR after training were observed in the antioxidant group than in the control group. (See Table 2. for other outcomes.) |
|
Mashhadi N.S. et al., 2018 [122] |
Randomized, double-blind, placebo-controlled, prospective study |
44 participants with type 2 diabetes |
0, 8 mg/day |
8 weeks |
Increased the serum adiponectin concentration, reduced visceral body fat mass (p < 0.01), serum triglyceride and VLDL chol concentrations, systolic blood pressure, fructosamine concentration (p < 0.05) and marginally reduced the plasma glucose concentration (p = 0.057). |
|
Canas J. A. et al., 2017 [121] |
Randomized, double-blind, placebo-controlled, prospective study |
20 children with simple obesity (BMI > 90%) |
500 μg/day * (MCS) |
6 months |
Mixed-carotenoid supplementation (MCS) increased β-carotene, total adiponectin, and high-molecular-weight adiponectin in plasma compared with placebo; MCS decreased BMI z-score, waist-to-height ratio, and subcutaneous adipose tissue compared with placebo. AX was used as a part of MCS. |
|
Takemoto M. et al., 2015 [123] |
Case report |
1 Werner syndrome patient |
12 mg/day * |
6 months |
Improved blood transaminase concentrations before AX intervention and 3 and 6 months after initiation were: AST 40 IU/L, 41 IU/L, and 20 IU/L; ALT 69 IU/L, 62 IU/L, and 34 IU/L; GGT 38 IU/L, 41 IU/L, and 35 IU/L; and cholinesterase 360 IU/L, 366 IU/L, and 331 IU/L, respectively. Liver-to-spleen Hounsfield units on CT were 0.41 before AX initiation, 0.71 at 3 months, and 0.94 at 6 months. No significant changes after AX intervention in hyaluronic acid, a marker of liver fibrosis; high-sensitivity C-reactive protein, a marker of inflammation; and MDA-modified LDL. |
|
Ni Y. et al., 2015[95] |
Randomized, single-blind, placebo-controlled, prospective study |
12 NASH patients |
12 mg/day |
24 weeks |
Improved steatosis (p < 0.05), marginally improved lobular inflammation (p = 0.15) and NAFLD activity score (p = 0.08) |
|
Choi H.D. et al., 2011 [38] |
Randomized, double-blind, placebo-controlled, prospective study |
27 overweight subjects (BMI >25.0 kg/m2) |
0, 20 mg/day |
12 weeks |
Decreased LDL chol and ApoB. (See Table 1. For other outcomes.) |
|
Yoshida H. et al., 2010 [124] |
Randomized, ouble-blind, placebo-controlled, prospective study |
61 non-obese subjects with fasting serum triglyceride of 120–200 mg/dL and without diabetes and hypertension |
0, 6, 12, 18 mg/day |
12 weeks |
Multiple comparison: triglycerides were significantly decreased by 12 and 18 mg/day and HDL-cholesterol was significantly increased by 6 and 12 mg. Serum adiponectin was increased by AX (12 and 18 mg/day), and changes in adiponectin were positively correlated with changes in HDL-chol. |
|
Satoh A. et al., 2009 [125] |
Open-label, prospective study |
20 subjects at risk for developing metabolic syndrome (from 127 healthy subjects) |
4, (8, 20) mg/day |
4 weeks. |
When subjects who met the diagnostic criteria for metabolic syndrome in Japan (SBP ≥ 130 mmHg, DBP ≥ 85 mmHg, TG ≥ 150 mg/dL, FG ≥ 100 mg/dL) at the start of the study were selected from 4 mg group, significant decreased in SBP(p < 0.01). On the other hand, there was no significant decrease in DBP. Reduced TG after treatment (218 mg/dL) than the baseline value (292 mg/dL), marginally reduced fasting glucose after the intervention (p < 0.1). |
|
Uchiyama A. et al., 2008 [126] |
Open-label, prospective study |
17 subjects at risk for developing metabolic syndrome |
8 mg twice day |
3 months |
Significant decreases plasma HbAlc (p = 0.0433) and TNF-α levels (p = 0.0022) and increase adiponectin concentration (p = 0.0053). N.S: body weight, BMI and waist circumference. |
|
Fukamauchi M. et al., 2007 [127] |
Randomized, double-blind, placebo-controlled, prospective study |
32 healthy subjects |
0, 6 mg/day |
6 weeks |
Synergistic effects of AX intake (12 mg/day, 6 weeks) and aerobic exercise (walking) were studied. AX contributed to reduction of body fat and suppressed the increase in blood lactate level after exercise. |
|
Kim Y.K. et al., 2004 [48] |
Open-label, prospective study |
15 healthy postmenopausal female subjects |
0, 2, 8 mg/day |
8 weeks |
Increase HDL-chol levels in 2 mg and 8 mg group increased significantly after 8 weeks from 50.6 ± 5.8 to 60.4 ± 7.1 mg/dL, 44.4 ± 10.7 to 49.4 ± 2.7 mg/dL respectively (p < 0.05). In the 2 mg group, triglyceride decreased significantly from 171.6 ± 67.4 mg/dL to 145.8 ± 5.1 mg/dL (p < 0.05). (See Table 1. For other outcomes.) |
* In addition to AX, other nutrients such as antioxidants were used in the study.
This entry is adapted from the peer-reviewed paper 10.3390/nu14010107
References
- Britton, G. Carotenoids. In Natural Food Colorants; Hendry, G.A.F., Houghton, J.D., Eds.; Springer: Boston, MA, USA, 1996; pp. 197–243.
- Britton, G.; Pfander, H.; Liaaen-Jensen, S. Carotenoids Volume 5: Nutrition and Health; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5.
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488.
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78.
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945.
- Misawa, N.; Takemura, M.; Maoka, T. Carotenoid Biosynthesis in Animals: Case of Arthropods. In Carotenoids Biosynthetic Biofunctional Approaches; Springer: Singapore, 2021; Volume 1261, pp. 217–220.
- Nishida, Y. Astaxanthin: Commercial production and its potential health-promoting effects. Oleoscience 2012, 12, 525–531.
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152.
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666.
- Sangeetha, R.K.; Baskaran, V. Retinol-deficient rats can convert a pharmacological dose of astaxanthin to retinol: Antioxidant potential of astaxanthin, lutein, and β-carotene. Can. J. Physiol. Pharmacol. 2010, 88, 977–985.
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Photo Protection of Haematococcus pluvialis Algae by Astaxanthin: Unique Properties of Astaxanthin Deduced by EPR, Optical and Electrochemical Studies. Antioxidants 2017, 6, 80.
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007, 11, 16–20.
- Ouchi, A.; Aizawa, K.; Iwasaki, Y.; Inakuma, T.; Terao, J.; Nagaoka, S.-I.; Mukai, K. Kinetic Study of the Quenching Reaction of Singlet Oxygen by Carotenoids and Food Extracts in Solution. Development of a Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Agric. Food Chem. 2010, 58, 9967–9978.
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 1996, 62, 134–137.
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146.
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of Low-Density Lipoprotein Oxidation by Astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222.
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618.
- Miyazawa, T.; Nakagawa, K.; Kimura, F.; Satoh, A.; Miyazawa, T. Erythrocytes carotenoids after astaxanthin supplementation in middle-aged and senior Japanese subjects. J. Oleo Sci. 2011, 60, 495–499.
- Matthews, S.J.; Ross, N.W.; Lall, S.P.; Gill, T.A. Astaxanthin binding protein in Atlantic salmon. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 144, 206–214.
- Kawasaki, S.; Mizuguchi, K.; Sato, M.; Kono, T.; Shimizu, H. A Novel Astaxanthin-Binding Photooxidative Stress-Inducible Aqueous Carotenoprotein from a Eukaryotic Microalga Isolated from Asphalt in Midsummer. Plant Cell Physiol. 2013, 54, 1027–1040.
- Nur-E-Borhan, S.A.; Okada, S.; Watabe, S.; Yamaguchi, K. Carotenoproteins from the Exoskeleton and the Muscular Epithelium of the Black Tiger Prawn Penaeus monodon. Fish. Sci. 1995, 61, 337–343.
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta Biomembr. 2001, 1512, 251–258.
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 167–174.
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta Biomembr. 1996, 1285, 167–174.
- Socaciu, C.; Jessel, R.; Diehl, H.A. Carotenoid incorporation into microsomes: Yields, stability and membrane dynamics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2799–2809.
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczyńska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orientation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619.
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262.
- Kobayashi, M.; Katsuragi, T.; Tani, Y. Enlarged and Astaxanthin-Accumulating Cyst Cells of the Green Alga Haematococcus pluvialis. J. Biosci. Bioeng. 2001, 92, 565–568.
- Aoi, W.; Naito, Y.; Sakuma, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokuni, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin Limits Exercise-Induced Skeletal and Cardiac Muscle Damage in Mice. Antioxid. Redox Signal. 2003, 5, 139–144.
- McAllister, M.J.; Mettler, J.A.; Patek, K.; Butawan, M.; Bloomer, R.J. Astaxanthin supplementation increases glutathione concentrations but does not impact fat oxidation during exercise in active young men. Int. J. Sport Nutr. Exerc. Metab. 2021, 1, 1–8.
- Petyaev, I.M.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Kyle, N.H.; Bashmakov, Y.K. Markers of Hypoxia and Oxidative Stress in Aging Volunteers Ingesting Lycosomal Formulation of Dark Chocolate Containing Astaxanthin. J. Nutr. Health Aging 2018, 22, 1092–1098.
- Chalyk, N.E.; Klochkov, V.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48.
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M. The effect of astaxanthin on vascular endothelial growth factor (VEGF) levels and peroxidation reactions in the aqueous humor. J. Clin. Biochem. Nutr. 2016, 59, 10–15.
- Baralic, I.; Andjelkovic, M.; Djordjevic, B.; Dikic, N.; Radivojevic, N.; Suzin-Zivkovic, V.; Radojevic-Skodric, S.; Pejic, S. Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–9.
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phytother. Res. 2012, 27, 1536–1542.
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7.
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of Astaxanthin on Oxidative Stress in Overweight and Obese Adults. Phytother. Res. 2011, 25, 1813–1818.
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369.
- Hashimoto, H.A.K.; Okamoto, Y.; Takahashi, J.; Chikuda, M.; Obara, Y. Effect of astaxanthin consumption on hydroper-oxides in the aqueous. Jpn. J. Clin. Ophthalmol. 2011, 65, 465–470.
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.; Shin, W.G. Protective Effects of Haematococcus Astaxanthin on Oxidative Stress in Healthy Smokers. J. Med. Food 2011, 14, 1469–1475.
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571.
- Peng, L.; Zhao, P.; Li, B.; Zhang, J.; Huang, C. Antioxidant effects and impact on human health of astaxanthin. Chin. J. Food Hyg. 2011, 23, 313–316.
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18.
- Iwabayashi, M.; Fujioka, N.; Nomoto, K.; Miyazaki, R.; Takahashi, H.; Hibino, S.; Takahashi, Y.; Nishikawa, K.; Nishida, M.; Yonei, Y. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti-Aging Med. 2009, 6, 15–21.
- Yamada, T.; Ryo, K.; Tai, Y.; Tamaki, Y.; Inoue, H.; Mishima, K.; Tsubota, K.; Saito, I. Evaluation of Therapeutic Effects of Astaxanthin on Impairments in Salivary Secretion. J. Clin. Biochem. Nutr. 2010, 47, 130–137.
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008, 9, 17.
- Karppi, J.; Rissanen, T.H.; Nyyssönen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11.
- Kim, Y.K.; Chyun, J.H. The Effects of Astaxanthin Supplements on Lipid Peroxidation and Antioxidant Status in Postmeno-pausal Women. Nutr. Sci. 2004, 7, 41–46.
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can Xanthophyll-Membrane Interactions Explain Their Selective Presence in the Retina and Brain? Foods 2016, 5, 7.
- Sakai, S.; Sugawara, T.; Matsubara, K.; Hirata, T. Inhibitory Effect of Carotenoids on the Degranulation of Mast Cells via Suppression of Antigen-induced Aggregation of High Affinity IgE Receptors. J. Biol. Chem. 2009, 284, 28172–28179.
- Manabe, Y.; Hirata, T.; Sugawara, T. Inhibitory Effect of Carotenoids on Ligand-induced Lipid Raft Translocation of Immunoreceptors. J. Oleo Sci. 2019, 68, 149–158.
- Palozza, P.; Barone, E.; Mancuso, C.; Picci, N. The protective role of carotenoids against 7-keto-cholesterol formation in solution. Mol. Cell. Biochem. 2007, 309, 61–68.
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of Divergent Effects of Astaxanthin on Insulin Signaling in L6 Cells. Endocrinology 2013, 154, 2600–2612.
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105.
- Suzuki, Y.; Ohgami, K.; Shiratori, K.; Jin, X.-H.; Ilieva, I.; Koyama, Y.; Yazawa, K.; Yoshida, K.; Kase, S.; Ohno, S. Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the NF-κB signaling pathway. Exp. Eye Res. 2006, 82, 275–281.
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2009, 49, 119–126.
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; De Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899.
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner. Exp. Dermatol. 2012, 21, 11–17.
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183.
- Farruggia, C.; Yang, Y.; Kim, B.; Pham, T.; Bae, M.; Park, Y.-K.; Lee, J.-Y. Astaxanthin Plays Anti-inflammatory and Antioxidant Effects by Inhibiting NFkB Nuclear Translocation and NOX2 Expression in Macrophages. FASEB J. 2015, 29, 603–608.
- Hara, K.; Hamada, C.; Wakabayashi, K.; Kanda, R.; Kaneko, K.; Horikoshi, S.; Tomino, Y.; Suzuki, Y. Scavenging of reactive oxygen species by astaxanthin inhibits epithelial–mesenchymal transition in high glucose-stimulated mesothelial cells. PLoS ONE 2017, 12, e0184332.
- Li, D.; Tong, W.; Liu, D.; Zou, Y.; Zhang, C.; Xu, W. Astaxanthin mitigates cobalt cytotoxicity in the MG-63 cells by modulating the oxidative stress. BMC Pharmacol. Toxicol. 2017, 18, 58.
- Sakai, S.; Nishida, A.; Ohno, M.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; Andoh, A. Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis. J. Clin. Biochem. Nutr. 2019, 64, 66–72.
- Kwak, M.S.; Lim, J.W.; Kim, H. Astaxanthin Inhibits Interleukin-6 Expression in Cerulein/Resistin-Stimulated Pancreatic Acinar Cells. Mediat. Inflamm. 2021, 2021, 5587297.
- Manabe, E.; Handa, O.; Naito, Y.; Mizushima, K.; Akagiri, S.; Adachi, S.; Takagi, T.; Kokura, S.; Maoka, T.; Yoshikawa, T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2007, 103, 1925–1937.
- Kowshik, J.; Baba, A.B.; Giri, H.; Reddy, G.D.; Dixit, M.; Nagini, S. Astaxanthin Inhibits JAK/STAT-3 Signaling to Abrogate Cell Proliferation, Invasion and Angiogenesis in a Hamster Model of Oral Cancer. PLoS ONE 2014, 9, e109114.
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203.
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374.
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31.
- Murphy, M.P. Mitochondrial Dysfunction Indirectly Elevates ROS Production by the Endoplasmic Reticulum. Cell Metab. 2013, 18, 145–146.
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13.
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2015, 23, 303–314.
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293.
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897.
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389.
- Sun, L.; Miyaji, N.; Yang, M.; Mills, E.M.; Taniyama, S.; Uchida, T.; Nikawa, T.; Li, J.; Shi, J.; Tachibana, K.; et al. Astaxanthin Prevents Atrophy in Slow Muscle Fibers by Inhibiting Mitochondrial Reactive Oxygen Species via a Mitochondria-Mediated Apoptosis Pathway. Nutrients 2021, 13, 379.
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38.
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418.
- Krestinin, R.; Baburina, Y.; Odinokova, I.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Krestinina, O. Isopro-terenol-Induced Permeability Transition Pore-Related Dysfunction of Heart Mitochondria Is Attenuated by Astaxanthin. Biomedicines 2020, 8, 437.
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxid. Med. Cell. Longev. 2020, 2020, 4629189.
- Sztretye, M.; Singlár, Z.; Szabó, L.; Angyal, Á.; Balogh, N.; Vakilzadeh, F.; Szentesi, P.; Dienes, B.; Csernoch, L. Improved Tetanic Force and Mitochondrial Calcium Homeostasis by Astaxanthin Treatment in Mouse Skeletal Muscle. Antioxidants 2020, 9, 98.
- García, F.; Lobos, P.; Ponce, A.; Cataldo, K.; Meza, D.; Farías, P.; Estay, C.; Oyarzun-Ampuero, F.; Herrera-Molina, R.; Paula-Lima, A.; et al. Astaxanthin Counteracts Excitotoxicity and Reduces the Ensuing Increases in Calcium Levels and Mitochondrial Reactive Oxygen Species Generation. Mar. Drugs 2020, 18, 335.
- Altunrende, M.E.; Gezen-Ak, D.; Atasoy, I.L.; Candas, E.; Dursun, E. The Role of Astaxanthin on Transcriptional Regulation of NMDA Receptors Voltage Sensitive Calcium Channels and Calcium Binding Proteins in Primary Cortical Neurons. Arch. Neuropsychiatry 2018, 55, 295–300.
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Astaxanthin Inhibits Glutamate Release in Rat Cerebral Cortex Nerve Terminals via Suppression of Voltage-Dependent Ca2+ Entry and Mitogen-Activated Protein Kinase Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8271–8278.
- Lin, X.; Zhao, Y.; Li, S. Astaxanthin attenuates glutamate-induced apoptosis via inhibition of calcium influx and endoplasmic reticulum stress. Eur. J. Pharmacol. 2017, 806, 43–51.
- Liu, P.H.; Aoi, W.; Takami, M.; Terajima, H.; Tanimura, Y.; Naito, Y.; Itoh, Y.; Yoshikawa, T. The astaxanthin-induced improvement in lipid metabolism during exercise is mediated by a PGC-1α increase in skeletal muscle. J. Clin. Biochem. Nutr. 2014, 54, 86–89.
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci. 2007, 80, 522–529.
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of Astaxanthin Supplementation on Exercise-Induced Fatigue in Mice. Biol. Pharm. Bull. 2006, 29, 2106–2110.
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachex Sarcopenia Muscle 2020, 11, 241–258.
- Naito, Y.; Uchiyama, K.; Mizushima, K.; Kuroda, M.; Akagiri, S.; Takagi, T.; Handa, O.; Kokura, S.; Yoshida, N.; Ichikawa, H.; et al. Microarray profiling of gene expression patterns in glomerular cells of astaxanthin-treated diabetic mice: A nutrigenomic approach. Int. J. Mol. Med. 2006, 18, 685–695.
- Miotto, P.M.; LeBlanc, P.J.; Holloway, G.P. High-Fat Diet Causes Mitochondrial Dysfunction as a Result of Impaired ADP Sensitivity. Diabetes 2018, 67, 2199–2205.
- Shi, S.Y.; Lu, S.-Y.; Sivasubramaniyam, T.; Revelo, X.; Cai, E.P.; Luk, C.T.; Schroer, S.A.; Patel, P.; Kim, R.; Bombardier, E.; et al. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 2015, 6, 7415.
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152.
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75.
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192.
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2010, 105, 220–227.
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of Astaxanthin inHaematococcusAlgal Extract: The Effects of Timing of Diet and Smoking Habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932.
- Coral-Hinostroza, G.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 99–110.
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304.
- Brown, D.R.; Warner, A.R.; Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. The effect of astaxanthin supplementation on performance and fat oxidation during a 40 km cycling time trial. J. Sci. Med. Sport 2020, 24, 92–97.
- Talbott, S.H.; Hantla, D.; Capelli, B.; Ding, L.; Li, Y.; Artaria, C. Effect of Astaxanthin Supplementation on Cardiorespiratory Function in Runners. EC Nutr. 2016, 11, 253–259.
- Klinkenberg, L.J.J.; Res, P.T.; Haenen, G.; Bast, A.; van Loon, L.J.; Van Dieijen-Visser, M.P.; Meex, S.J. Effect of Antioxidant Supplementation on Exercise-Induced Cardiac Troponin Release in Cyclists: A Randomized Trial. PLoS ONE 2013, 8, e79280.
- Res, P.T.; Cermak, N.M.; Stinkens, R.; Tollakson, T.J.; Haenen, G.; Bast, A.; van Loon, L.J. Astaxanthin Supplementation Does Not Augment Fat Use or Improve Endurance Performance. Med. Sci. Sports Exerc. 2013, 45, 1158–1165.
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52.
- Earnest, C.; Lupo, M.; White, K.; Church, T. Effect of Astaxanthin on Cycling Time Trial Performance. Int. J. Sports Med. 2011, 32, 882–888.
- Bloomer, R.J.; Fry, A.; Schilling, B.; Chiu, L.; Hori, N.; Weiss, L. Astaxanthin Supplementation Does Not Attenuate Muscle Injury Following Eccentric Exercise in Resistance-Trained Men. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 401–412.
- Sawaki, K.; Yoshigi, H.; Aoki, K.; Koikawa, N.; Azumane, A.; Kaneko, K.; Yamaguchi, M. Sports Performance Benefits from Taking Natural Astaxanthin Characterized by Visual Acuity and Muscle Fatigue Improvement in Humans. J. Clin. Ther. Med. 2002, 18, 1085–1100.
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Kuwahata, M.; Higashi, A. Astaxanthin-, β-Carotene-, and Resveratrol-Rich Foods Support Resistance Training-Induced Adaptation. Antioxidants 2021, 10, 113.
- Fleischmann, C.; Horowitz, M.; Yanovich, R.; Raz, H.; Heled, Y. Asthaxanthin Improves Aerobic Exercise Recovery Without Affecting Heat Tolerance in Humans. Front. Sports Act. Living 2019, 1, 17.
- Takami, M.; Aoi, W.; Terajima, H.; Tanimura, Y.; Wada, S.; Higashi, A. Effect of dietary antioxidant-rich foods combined with aerobic training on energy metabolism in healthy young men. J. Clin. Biochem. Nutr. 2019, 64, 79–85.
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2018, 10, 281.
- Hongo, N. Daily Fatigue—reducing Effect of Astaxanthin―A Randomized, Placebo—controlled, Double—blind, Par-allel—group Study. Jpn. Pharmacol. Ther. 2017, 45, 61–72.
- Malmstena, C.L.; Lignell, A. Dietary Supplementation with Astaxanthin-Rich Algal Meal Improves Strength Endurance—A Double Blind Placebo Controlled Study on Male Students. Carotenoid Sci. 2008, 13, 20–22.
- Tajima, T.; Nagata, A. Effects of astaxanthin ingestion on exercise-induced physiological changes. Health Behav. Sci. 2004, 3, 5–10.
- Liu, S.Z.; Valencia, A.P.; VanDoren, M.P.; Shankland, E.G.; Roshanravan, B.; Conley, K.E.; Marcinek, D.J. Astaxanthin supplementation enhances metabolic adaptation with aerobic training in the elderly. Physiol. Rep. 2021, 9, e14887.
- Liu, S.Z.; Ali, A.S.; Campbell, M.D.; Kilroy, K.; Shankland, E.G.; Roshanravan, B.; Marcinek, D.J.; Conley, K.E. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachexia Sarcopenia Muscle 2018, 9, 826–833.
- Fujino, H.; Kondo, H.; Kanazashi, M.; Nakanishi, R.; Tanaka, M.; Ishihara, A. Dietary Astaxanthin Supplementation Improves Walking Performance and Blood Lactate Level After Walking Test in Community-dwelling Elderly Subjects: 453 Board #290 June 1, 9:30 AM—11:00 AM. Med. Sci. Sports Exerc. 2016, 48, 129.
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14022.
- Kato, T.; Kasai, T.; Sato, A.; Ishiwata, S.; Yatsu, S.; Matsumoto, H.; Shitara, J.; Murata, A.; Shimizu, M.; Suda, S.; et al. Effects of 3-Month Astaxanthin Supplementation on Cardiac Function in Heart Failure Patients with Left Ventricular Systolic Dysfunction—A Pilot Study. Nutrients 2020, 12, 1896.
- Chan, K.-C.; Chen, S.-C.; Chen, P.-C. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J. Funct. Foods 2018, 53, 22–27.
- Canas, J.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990.
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin im-proves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346.
- Takemoto, M.; Yamaga, M.; Furuichi, Y.; Yokote, K. Astaxanthin Improves Nonalcoholic Fatty Liver Disease in Werner Syndrome with Diabetes Mellitus. J. Am. Geriatr. Soc. 2015, 63, 1271–1273.
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523.
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of a New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284.
- Uchiyama, A. Clinical efficacy of astaxanthin-containing Haematococcus pluvialis extract for the volunteers at risk of metabolic syndrome. J. Clin. Biochem. Nutr. 2008, 43, 38–43.
- Fukamauchi, M. Food functionality of astaxanthin-10: Synergistic effects of astaxanthin intake and aerobic exercise. Food Style 2007, 11, 22–24.
