Encapsulation is defined as the process to entrap one substance (active agent) within another substance, yielding small particles that release their contents at controlled rates over prolonged periods of time and under specific conditions. Antimicrobial active packaging has emerged as an effective technology to reduce microbial growth in food products increasing both their shelf-life and microbial safety for the consumer while maintaining their quality and sensorial properties.
- active packaging
- antimicrobials
- encapsulation
- electrospinning
- nanocarriers
- essential oils
- metal nanoparticles
- emulsions
- natural compounds
1. Antimicrobial Food Packaging
1.1. Antimicrobial Substances Used in Food Packaging
|
Antimicrobial Class |
Antimicrobial Agent |
Packaging Material |
Main Microorganisms |
Food Product |
Ref. |
|---|---|---|---|---|---|
|
Organic acids |
Lactic acid |
Polyamide |
Escherichia coli O157:H7 |
Fresh beef cuts |
[10] |
|
Lactic acid |
Chitosan pectin starch biocomposite |
Bacillus subtilis Listeria monocytogenes |
NA |
[11] |
|
|
Sodium benzoate Citric acid |
Polyvinyl alcohol (PVA) |
Staphylococcus aureus Escherichia coli Candida albicans |
NA |
[12] |
|
|
Potassium sorbate |
Fish collagen and polyvinyl alcohol (PVA) composite |
Escherichia coli Staphylococcus aureus |
NA |
[13] |
|
|
Bacteriocins |
Sakacin-A |
PE coated paper |
Listeria monocytogenes |
Thin-cut meat |
[14] |
|
Sakacin-A |
Cellulose nanofibres |
Listeria monocytogenes |
Smoked salmon fillets |
[15] |
|
|
Nisin |
Starch-halloysite nanocomposites |
Listeria monocytogenes Clostridium perfringens |
NA |
[16] |
|
|
Pediocin |
Starch-halloysite nanocomposites |
Listeria monocytogenes Clostridium perfringens |
NA |
[16] |
|
|
Nisin |
Chitosan-carboxymethylchitosan composite films |
Listeria monocytogenes |
NA |
[17] |
|
|
Bacteriocin 7293 |
Poly (lactic acid)/sawdust particle biocomposite film |
Listeria monocytogenes Staphylococcus aureus Pseudomonas aeruginosa Aeromonas hydrophila Escherichia coli Salmonella Typhimurium |
Pangasius fish fillets |
[18] |
|
|
Bacteriocin-like substances |
Starch |
Listeria monocytogenes |
Cheese |
[19] |
|
|
Bacteriocin-like substances |
Triticale flour films |
Listeria innocua |
Cheese |
[20] |
|
|
Bacteriocin-producer living bacteria |
Poly (ethylene terephthalate) (PET) coated with polyvinyl alcohol (PVOH) |
Listeria monocytogenes |
Precooked chicken fillets |
[21] |
|
|
Enzymes |
Lysozyme |
Nonwoven regenerated cellulose with carbon nanotubes and graphene oxide |
Micrococcus lysodeikticus |
NA |
[22] |
|
Lysozyme+ lactoferrin |
Carboxymethyl cellulose-coated paper |
Listeria innocua Escherichia coli |
Veal carpaccio |
[23] |
|
|
Lysozyme |
Polyamide 11 (PA11) with halloysite nanotubes (HNTs) |
Pseudomonads |
Chicken slices |
[24] |
|
|
Glucose oxidase |
Whey protein isolate |
Listeria innocua Brochothrix thermosphacta Escherichia coli Enterococcus faecalis |
NA |
[25] |
|
|
Lactoperoxidase |
Chitosan |
Shewanella putrefaciens Pseudomonas fluorescens Psychrotrophs Mesophiles |
Rainbow trout |
[26] |
|
|
Biopolymers |
Chitosan |
Chitosan/ethylene copolymer |
Escherichia coli Salmonella Enteritidis Listeria monocytogenes |
NA |
[27] |
|
Hydroxyethyl cellulose/sodium alginate |
NA |
Escherichia coli Staphylococcus aureus |
NA |
[28] |
|
|
Bacteriophages |
ϕIBB-PF7A |
Alginate |
Pseudomonas fluorescens |
Chicken fillets |
[29] |
|
vB_EcoMH2W |
Chitosan |
Escherichia coli O157:H7 |
Tomatoes |
[30] |
|
|
LISTEX™ P100 |
Cellulose membranes |
Listeria monocytogenes |
Ready-to-eat turkey |
[31] |
|
|
Other |
LAE |
Cellulose nanofibres |
Listeria monocytogenes |
NA |
[32] |
|
Sulphur nanoparticles |
Chitosan |
Listeria monocytogenes Escherichia coli |
NA |
[33] |
|
|
Chlorine dioxide |
PLA |
Staphylococcus aureus Escherichia coli |
NA |
[34] |
|
|
Quaternary ammonium salt |
PVA/starch |
Staphylococcus aureus Bacillus subtilis Escherichia coli Pseudomonas aeruginosa |
NA |
[35] |
2. Encapsulation Strategies for Antimicrobial Packaging
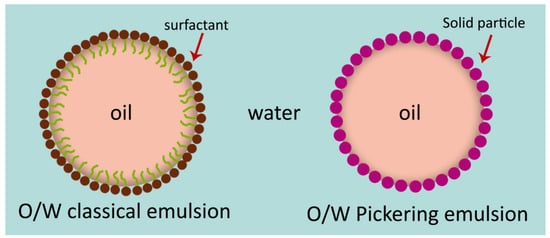
2.1. Emulsions
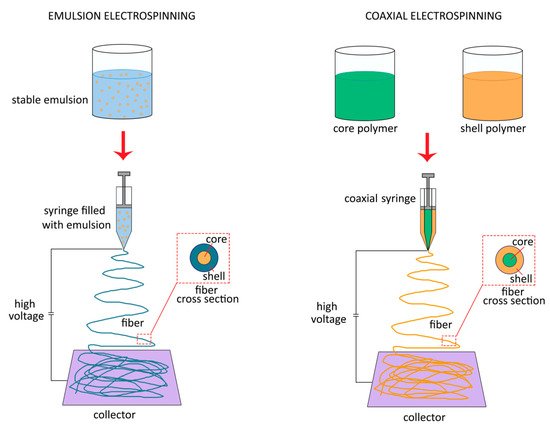
2.2. Core-Shell Nanofibers: Emulsion and Coaxial Electrospinning
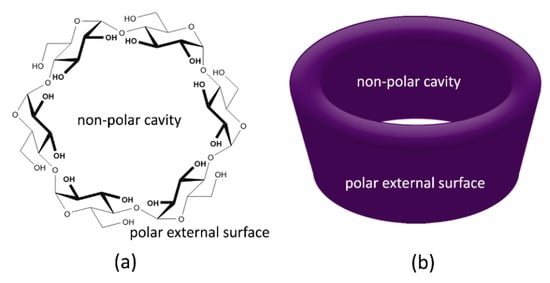
2.3. Cyclodextrins
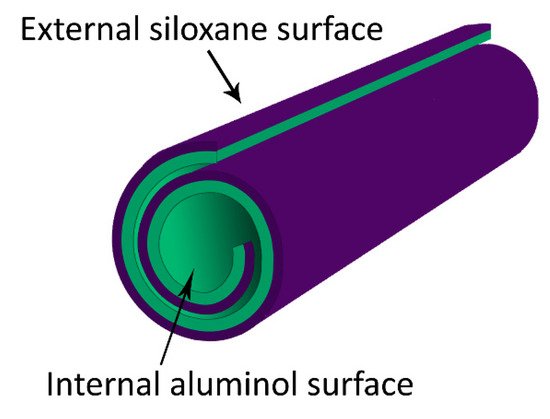
2.4. Halloysites Nanotubes
2.5. Liposomes
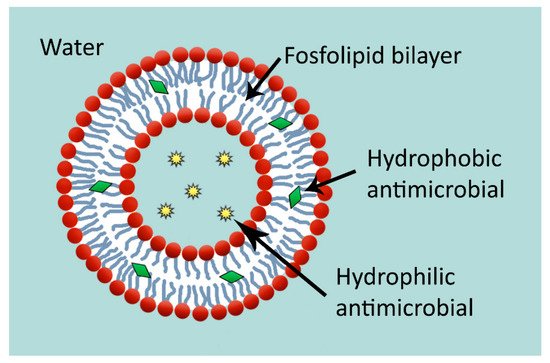
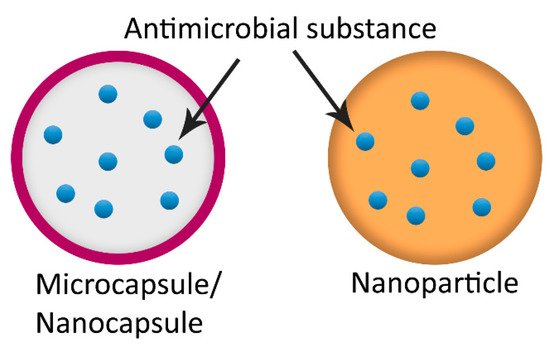
2.6. Other Encapsulating Particles
This entry is adapted from the peer-reviewed paper 10.3390/molecules25051134
References
- European Union Food Safety to Fork: Safe and Healthy Food for Everyone The EU Explained, Agriculture. Available online: http://europa.eu/pol/index_en.htm (accessed on 1 March 2020).
- European Parliament Legislative Resolution of 19 November 2008 on the Proposal for A Council Decision Amending Decision 2006/144/EC on the Community Strategic Guidelines for Rural Development (Programming Period 2007 to 2013). Off. J. Eur. Union 2013. Available online: https://op.europa.eu/en/publication-detail/-/publication/464b67b4-2521-459a-a2f0-2520b8783d07/language-en (accessed on 20 January 2020).
- Key Facts on Food Loss and Waste You Should Know! | SAVE FOOD: Global Initiative on Food Loss and Waste Reduction | Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/save-food/resources/keyfindings/en/ (accessed on 11 November 2019).
- Lemaire, A.; Limbourg, S. How can food loss and waste management achieve sustainable development goals? J. Clean. Prod. 2019, 234, 1221–1234.
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73.
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization (WHO): Geneva, Switzerland, 2015; pp. 1–15.
- Radusin, T.; Škrinjar, M.M.; Cabarkapa, I.; Pilić, B.; Novaković, A.R.; Hromiš, N.M. Actual and future trends in antimicrobial food packaging. Agro Food Ind. Hi. Tech. 2013, 24, 44–48.
- Silva, F.; Becerril, R.; Nerin, C. Safety assessment of active food packaging: Role of known and unknown substances. In Advances in the Determination of Xenobiotics in Foods; Bentham Science Publishers Pte. Ltd.: Singapore, 2019; pp. 1–41.
- Dobrucka, R. Antimicrobial packaging with natural compunds - a review. Logforum 2015, 12, 193–202.
- Smulders, F.J.M.; Paulsen, P.; Vali, S.; Wanda, S. Effectiveness of a polyamide film releasing lactic acid on the growth of E. coli O157: H7, Enterobacteriaceae and Total Aerobic Count on vacuum-packed beef. Meat Sci. 2013, 95, 160–165.
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255.
- Birck, C.; Degoutin, S.; Maton, M.; Neut, C.; Bria, M.; Moreau, M.; Fricoteaux, F.; Miri, V.; Bacquet, M. Antimicrobial citric acid/poly(vinyl alcohol) crosslinked films: Effect of cyclodextrin and sodium benzoate on the antimicrobial activity. Lwt - Food Sci. Technol. 2016, 68, 27–35.
- Liang, X.; Feng, S.; Ahmed, S.; Qin, W.; Liu, Y. Effect of potassium sorbate and ultrasonic treatment on the properties of fish scale collagen/polyvinyl alcohol composite film. Molecules 2019, 24, 2363.
- Barbiroli, A.; Musatti, A.; Capretti, G.; Iametti, S.; Rollini, M. Sakacin-A antimicrobial packaging for decreasing Listeria contamination in thin-cut meat: Preliminary assessment. J. Sci. Food Agric. 2017, 97, 1042–1047.
- Mapelli, C.; Musatti, A.; Barbiroli, A.; Saini, S.; Bras, J.; Cavicchioli, D.; Rollini, M. Cellulose nanofiber (CNF)–sakacin-A active material: Production, characterization and application in storage trials of smoked salmon. J. Sci. Food Agric. 2019, 99, 4731–4738.
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561–570.
- Zimet, P.; Mombrú, Á.W.; Mombrú, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343.
- Woraprayote, W.; Kingcha, Y.; Amonphanpokin, P.; Kruenate, J.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Anti-listeria activity of poly(lactic acid)/sawdust particle biocomposite film impregnated with pediocin PA-1/AcH and its use in raw sliced pork. Int. J. Food Microbiol. 2013, 167, 229–235.
- de Lima Marques, J.; Funck, G.D.; da Silva Dannenberg, G.; dos Santos Cruxen, C.E.; El Halal, S.L.M.; Dias, A.R.G.; Fiorentini, Â.M.; da Silva, W.P. Bacteriocin-like substances of Lactobacillus curvatus P99: Characterization and application in biodegradable films for control of Listeria monocytogenes in cheese. Food Microbiol. 2017, 63, 159–163.
- Salvucci, E.; Rossi, M.; Colombo, A.; Pérez, G.; Borneo, R.; Aguirre, A. Triticale flour films added with bacteriocin-like substance (BLIS) for active food packaging applications. Food Packag. Shelf Life 2019, 19, 193–199.
- Degli Esposti, M.; Toselli, M.; Sabia, C.; Messi, P.; de Niederhäusern, S.; Bondi, M.; Iseppi, R. Effectiveness of polymeric coated films containing bacteriocin-producer living bacteria for Listeria monocytogenes control under simulated cold chain break. Food Microbiol. 2018, 76, 173–179.
- Liu, Y.; Vincent Edwards, J.; Prevost, N.; Huang, Y.; Chen, J.Y. Physico- and bio-activities of nanoscale regenerated cellulose nonwoven immobilized with lysozyme. Mater. Sci. Eng. C 2018, 91, 389–394.
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392.
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial membranes of bio-based pa 11 and hnts filled with lysozyme obtained by an electrospinning process. Nanomaterials 2018, 8, 47–53.
- Murillo-Martínez, M.M.; Tello-Solís, S.R.; García-Sánchez, M.A.; Ponce-Alquicira, E. Antimicrobial Activity and Hydrophobicity of Edible Whey Protein Isolate Films Formulated with Nisin and/or Glucose Oxidase. J. Food Sci. 2013, 78, M560–M566.
- Jasour, M.S.; Ehsani, A.; Mehryar, L.; Naghibi, S.S. Chitosan coating incorporated with the lactoperoxidase system: An active edible coating for fish preservation. J. Sci. Food Agric. 2015, 95, 1373–1378.
- Massouda, D.F.; Visioli, D.; Green, D.A.; Joerger, R.D. Extruded blends of chitosan and ethylene copolymers for antimicrobial packaging. Packag. Technol. Sci. 2012, 25, 321–327.
- Şen, F.; Kahraman, M.V. Preparation and characterization of hybrid cationic hydroxyethyl cellulose/sodium alginate polyelectrolyte antimicrobial films. Polym. Adv. Technol. 2018, 29, 1895–1901.
- Alves, D.; Marques, A.; Milho, C.; José Costa, M.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. Int. J. Food Microbiol. 2018, 291, 121–127.
- Amarillas, L.; Lightbourn-Rojas, L.; Angulo-Gaxiola, A.K.; Basilio Heredia, J.; González-Robles, A.; León-Félix, J. The antibacterial effect of chitosan-based edible coating incorporated with a lytic bacteriophage against Escherichia coli O157:H7 on the surface of tomatoes. J. Food Saf. 2018, 38, e12571–e12581.
- Lone, A.; Anany, H.; Hakeem, M.; Aguis, L.; Avdjian, A.-C.; Bouget, M.; Atashi, A.; Brovko, L.; Rochefort, D.; Griffiths, M.W. Development of prototypes of bioactive packaging materials based on immobilized bacteriophages for control of growth of bacterial pathogens in foods. Int. J. Food Microbiol. 2016, 217, 49–58.
- Silva, F.; Gracia, N.; McDonagh, B.H.; Domingues, F.C.; Nerín, C.; Chinga-Carrasco, G. Antimicrobial activity of biocomposite films containing cellulose nanofibrils and ethyl lauroyl arginate. J. Mater. Sci. 2019, 54, 12159–12170.
- Shankar, S.; Rhim, J.W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123.
- Huang, C.; Zhang, B.; Wang, S.; Zhang, L.; Wang, J.; Huang, X.; Zhao, Y.; Huang, L. Moisture-triggered release of self-produced ClO2 gas from microcapsule antibacterial film system. J. Mater. Sci. 2018, 53, 12704–12717.
- Sekhavat Pour, Z.; Makvandi, P.; Ghaemy, M. Performance properties and antibacterial activity of crosslinked films of quaternary ammonium modified starch and poly(vinyl alcohol). Int. J. Biol. Macromol. 2015, 80, 596–604.
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815.
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60.
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11.
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation techniques for compounds and products with antioxidant and antimicrobial activity - A critical view. Eur. J. Med. Chem. 2018, 157, 1326–1345.
- Espitia, P.J.P.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, Characterization, and Application in Bio-Based Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 264–285.
- Robledo, N.; López, L.; Bunger, A.; Tapia, C.; Abugoch, L. Effects of antimicrobial edible coating of thymol nanoemulsion/quinoa protein/chitosan on the safety, sensorial properties, and quality of refrigerated strawberries (Fragaria × ananassa) under commercial storage environment. Food Bioprocess Technol. 2018, 11, 1566–1574.
- Frank, K.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Alginate biocomposite films incorporated with cinnamon essential oil nanoemulsions: Physical, mechanical, and antibacterial properties. Int. J. Polym. Sci. 2018, 2018, 1519408–1519416.
- Hashemi Gahruie, H.; Ziaee, E.; Eskandari, M.H.; Hosseini, S.M.H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017, 166, 93–103.
- Jantrawut, P.; Boonsermsukcharoen, K.; Thipnan, K.; Chaiwarit, T.; Hwang, K.-M.; Park, E.-S. Enhancement of Antibacterial Activity of Orange Oil in Pectin Thin Film by Microemulsion. Nanomaterials 2018, 8, 545.
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671.
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729.
- Guo, M.; Jin, T.Z.; Yadav, M.P.; Yang, R. Antimicrobial property and microstructure of micro-emulsion edible composite films against Listeria. Int. J. Food Microbiol. 2015, 208, 58–64.
- Guo, M.; Yadav, M.P.; Jin, T.Z. Antimicrobial edible coatings and films from micro-emulsions and their food applications. Int. J. Food Microbiol. 2017, 263, 9–16.
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de F.F. Soares, N.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194.
- Oh, Y.A.; Oh, Y.J.; Song, A.Y.; Won, J.S.; Song, K.B.; Min, S.C. Comparison of effectiveness of edible coatings using emulsions containing lemongrass oil of different size droplets on grape berry safety and preservation. LWT 2017, 75, 742–750.
- Fu, Y.; Sarkar, P.; Bhunia, A.K.; Yao, Y. Delivery systems of antimicrobial compounds to food. Trends Food Sci. Technol. 2016, 57, 165–177.
- Taştan, Ö.; Pataro, G.; Donsì, F.; Ferrari, G.; Baysal, T. Decontamination of fresh-cut cucumber slices by a combination of a modified chitosan coating containing carvacrol nanoemulsions and pulsed light. Int. J. Food Microbiol. 2017, 260, 75–80.
- Sugumar, S.; Mukherjee, A.; Chandrasekaran, N. Eucalyptus oil nanoemulsion-impregnated chitosan film: Antibacterial effects against a clinical pathogen, Staphylococcus aureus, in vitro. Int. J. Nanomed. 2015, 10, 67–75.
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12.
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320.
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 2018, 83, 445–453.
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177.
- Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polym. (Basel). 2019, 11, 1418.
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 2019, 106, 164–171.
- Amiri, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R. Combined antioxidant and sensory effects of corn starch films with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on fresh ground beef patties. Meat Sci. 2019, 153, 66–74.
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.; Amiri, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2018, 42, e13441.
- Hossain, F.; Follett, P.; Salmieri, S.; Vu, K.D.; Fraschini, C.; Lacroix, M. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int. J. Food Microbiol. 2019, 295, 33–40.
- Lei, K.; Wang, X.; Li, X.; Wang, L. The innovative fabrication and applications of carvacrol nanoemulsions, carboxymethyl chitosan microgels and their composite films. Colloids Surf. B Biointerfaces 2019, 175, 688–696.
- Ghani, S.; Barzegar, H.; Noshad, M.; Hojjati, M. The preparation, characterization and in vitro application evaluation of soluble soybean polysaccharide films incorporated with cinnamon essential oil nanoemulsions. Int. J. Biol. Macromol. 2018, 112, 197–202.
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Antibacterial hydroxypropyl methyl cellulose edible films containing nanoemulsions of Thymus daenensis essential oil for food packaging. Carbohydr. Polym. 2017, 175, 241–248.
- Gundewadi, G.; Rudra, S.G.; Sarkar, D.J.; Singh, D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packag. Shelf Life 2018, 18, 1–12.
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Phys. Eng. Asp. 2013, 439, 23–34.
- Almasi, H.; Azizi, S.; Amjadi, S. Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll. 2020, 99, 105338.
- Fasihi, H.; Fazilati, M.; Hashemi, M.; Noshirvani, N. Novel carboxymethyl cellulose-polyvinyl alcohol blend films stabilized by Pickering emulsion incorporation method. Carbohydr. Polym. 2017, 167, 79–89.
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186.
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. Rsc Adv. 2017, 7, 28951–28964.
- Zhang, H.; Hortal, M.; Dobon, A.; Jorda-Beneyto, M.; Bermudez, J.M. Selection of Nanomaterial-Based Active Agents for Packaging Application: Using Life Cycle Assessment (LCA) as a Tool. Packag. Technol. Sci. 2017, 30, 575–586.
- Zhao, X.; Lui, Y.S.; Wen, P.; Toh, J.; Chye, S.; Loo, J. Sustained Release of Hydrophilic L-ascorbic acid 2-phosphate Magnesium from Electrospun Polycaprolactone Scaffold-A Study across Blend, Coaxial, and Emulsion Electrospinning Techniques. Mater. (Basel). 2014, 7, 7398–7408.
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135, 46265.
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640.
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326.
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124.
- Chen, G.; Liu, B. Cellulose sulfate based film with slow-release antimicrobial properties prepared by incorporation of mustard essential oil and β-cyclodextrin. Food Hydrocoll. 2016, 55, 100–107.
- Mallardo, S.; De Vito, V.; Malinconico, M.; Volpe, M.G.; Santagata, G.; Di Lorenzo, M.L. Poly(butylene succinate)-based composites containing β-cyclodextrin/d-limonene inclusion complex. Eur. Polym. J. 2016, 79, 82–96.
- Samperio, C.; Boyer, R.; Eigel, W.N.; Holland, K.W.; McKinney, J.S.; O’Keefe, S.F.; Smith, R.; Marcy, J.E. Enhancement of Plant Essential Oils’ Aqueous Solubility and Stability Using Alpha and Beta Cyclodextrin. J. Agric. Food Chem. 2010, 58, 12950–12956.
- Wen, P.; Wen, Y.; Zong, M.-H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179.
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004.
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162.
- Simionato, I.; Domingues, F.C.; Nerín, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Carbohydr Polym 2018. submitted.
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Food Chem 2018. submitted.
- Du, M.; Guo, B.; Jia, D. Newly emerging applications of halloysite nanotubes: A review. Polym. Int. 2010, 59, 574–582.
- Hanif, M.; Jabbar, F.; Sharif, S.; Abbas, G.; Farooq, A.; Aziz, M. Halloysite nanotubes as a new drug-delivery system: A review. Clay Min. 2016, 51, 469–477.
- Zhang, H. Selective modification of inner surface of halloysite nanotubes: A review. Nanotechnol. Rev. 2017, 6, 573–581.
- Lvov, Y.M.; Shchukin, D.G.; Mö, H.; Price, R.R.; Muir, G. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano. 2008, 2, 814–820.
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017, 93, 495–506.
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53.
- Jang, S.S.; Jang, S.S.; Lee, G.; Ryu, J.; Park, S.; Park, N. Halloysite Nanocapsules Containing Thyme Essential Oil: Preparation, Characterization, and Application in Packaging Materials. J. Food Sci. 2017, 82, 2113–2120.
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126.
- Shemesh, R.; Krepker, M.; Nitzan, N.; Vaxman, A.; Segal, E. Active packaging containing encapsulated carvacrol for control of postharvest decay. Postharvest Biol. Technol. 2016, 118, 175–182.
- Maruthupandy, M.; Seo, J. Allyl isothiocyanate encapsulated halloysite covered with polyacrylate as a potential antibacterial agent against food spoilage bacteria. Mater. Sci. Eng. C 2019, 105, 110016–110025.
- Buendía−Moreno, L.; Sánchez−Martínez, M.J.; Antolinos, V.; Ros−Chumillas, M.; Navarro−Segura, L.; Soto−Jover, S.; Martínez−Hernández, G.B.; López−Gómez, A. Active cardboard box with a coating including essential oils entrapped within cyclodextrins and/or hallosyte nanotubes: A Case Study Fresh Tomato Storage. Food Control 2020, 107, 106763–106773.
- Alkan Tas, B.; Sehit, E.; Erdinc Tas, C.; Unal, S.; Cebeci, F.C.; Menceloglu, Y.Z.; Unal, H. Carvacrol loaded halloysite coatings for antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 20, 100300–100306.
- Lee, M.H.; Seo, H.-S.; Park, H.J. Thyme Oil Encapsulated in Halloysite Nanotubes for Antimicrobial Packaging System. J. Food Sci. 2017, 82, 922–932.
- Hallaj-Nezhadi, S.; Hassan, M. Nanoliposome-based antibacterial drug delivery. Drug Deliv. 2015, 22, 581–589.
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115.
- Valencia-Sullca, C.; Jiménez, M.; Jiménez, A.; Atarés, L.; Vargas, M.; Chiralt, A. Influence of liposome encapsulated essential oils on properties of chitosan films. Polym. Int. 2016, 65, 979–987.
- Wu, J.; Liu, H.; Ge, S.; Wang, S.; Qin, Z.; Chen, L.; Zheng, Q.; Liu, Q.; Zhang, Q. The preparation, characterization, antimicrobial stability and invitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocoll. 2015, 43, 427–435.
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192.
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164.
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450.
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718.
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134.
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27.
- Pu, C.; Tang, W. A chitosan-coated liposome encapsulating antibacterial peptide, Apep10: Characterisation, triggered-release effects and antilisterial activity in thaw water of frozen chicken. Food Funct. 2016, 7, 4310–4322.
- Cui, H.Y.; Wu, J.; Lin, L. Inhibitory effect of liposome-entrapped lemongrass oil on the growth of Listeria monocytogenes in cheese. J. Dairy Sci. 2016, 99, 6097–6104.
- Lin, L.; Zhang, X.; Zhao, C.; Cui, H. Liposome containing nutmeg oil as the targeted preservative against Listeria monocytogenes in dumplings. Rsc Adv. 2016, 6, 978–986.
- Lin, L.; Dai, Y.; Cui, H. Antibacterial poly(ethylene oxide) electrospun nanofibers containing cinnamon essential oil/beta-cyclodextrin proteoliposomes. Carbohydr. Polym. 2017, 178, 131–140.
- Sarkar, P.; Choudhary, R.; Panigrahi, S.; Syed, I.; Sivapratha, S.; Dhumal, C.V. Nano-inspired systems in food technology and packaging. Environ. Chem. Lett. 2017, 15, 607–622.
- Šumiga; Šumiga; Ravnjak; Boh Podgornik Antimicrobial Paper Coatings Containing Microencapsulated Cymbopogon citratus Oil. Coatings 2019, 9, 470.
- Marturano, V.; Marcille, H.; Cerruti, P.; Bandeira, N.A.; Giamberini, M.; Trojanowska, A.; Tylkowski, B.; Carfagna, C.; Ausanio, G.; Ambrogi, V. Visible-Light Responsive Nanocapsules for Wavelength-Selective Release of Natural Active Agents. Acs Appl. Nano Mater. 2019, 2, 4499–4506.
- Marturano, V.; Bizzarro, V.; Ambrogi, V.; Cutignano, A.; Tommonaro, G.; Abbamondi, G.R.; Giamberini, M.; Tylkowski, B.; Carfagna, C.; Cerruti, P. Light-Responsive Nanocapsule-Coated Polymer Films for Antimicrobial Active Packaging. Polym. (Basel). 2019, 11, 68.
- Zhang, B.; Huang, C.; Zhang, L.; Wang, J.; Huang, X.; Zhao, Y.; Liu, Y.; Li, C. Application of chlorine dioxide microcapsule sustained-release antibacterial films for preservation of mangos. J. Food Sci. Technol. 2019, 56, 1095–1103.
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198.
- Caro, N.; Medina, E.; Díaz-Dosque, M.; López, L.; Abugoch, L.; Tapia, C. Novel active packaging based on films of chitosan and chitosan/quinoa protein printed with chitosan-tripolyphosphate-thymol nanoparticles via thermal ink-jet printing. Food Hydrocoll. 2016, 52, 520–532.
- Cui, H.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78.
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93.
- Wu, C.; Zhu, Y.; Wu, T.; Wang, L.; Yuan, Y.; Chen, J.; Hu, Y.; Pang, J. Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging. Food Chem. 2019, 288, 139–145.
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun antimicrobial films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227.
- Tsai, Y.H.; Yang, Y.N.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296.
- Basu, A.; Kundu, S.; Sana, S.; Halder, A.; Abdullah, M.F.; Datta, S.; Mukherjee, A. Edible nano-bio-composite film cargo device for food packaging applications. Food Packag. Shelf Life 2017, 11, 98–105.
