Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Weaning affects the development of ruminal bacteria in lambs during early life. However, the temporal dynamics of rumen microbiota in early weaned lambs is unknown compared to conventionally weaned lambs. Early weaning led to a significant decrease in rumen microbiota richness and diversity in the short term. The changes in rumen microbiota are associated with the persistence of weaning stress.
- lamb
- early weaning
- rumen microbiota
- rumen development
- rumen maturation
- weaning stress
1. Introduction
Rumen, as a vital organ, plays a critical role in livestock. The structure and function of the rumen develops rapidly in the early stages of life, such as non-rumination, transition, and rumination. Previous studies have shown that early weaning can promote the early development of rumen structure and function [1,2]. For young ruminants, rumen maturation and its microbiota evolution are directly related to health and performance. Many factors influence microbial colonization, such as the dam’s vaginal microbiome and the types of microbes in the surrounding environment [3,4,5]. Feeding modes also affect the direct transmission of bacteria from the mother and the environment to newborns [6]. As rumen develops, its microbial community changes. Moreover, factors including weaning, feeding strategies, solid feed, starter composition, etc. can affect the early establishment and maturation of ruminal microbiota of lambs [2,7,8,9,10]. Weaning is one of the most stressful events in the life of a newborn. Commercial farms often separate ewes and lambs before natural weaning. The early separation results in the breaking of the ewe–lamb bond, with changes occurring in both the physical and social environment at the same time, combining also with the end of suckling and the complete replacement of milk by solid food. The latest research shows that weaning age affects the development of the ruminal bacteria in lambs during early life [2]. Other research has shown that weaning significantly influences the morphological and functional development of the rumen, and bacterial community composition [11].
In a previous study, lambs could be weaned at as early as 10 d using a liquid milk replacer [12]. Using a starter to wean lambs is practical in many studies [11,13]. The different weaning strategies result in different levels of animal performance and profit. However, there is no doubt that early weaning will cause a drop in feed consumption and a decrease in the growth rate of lambs in a short period [14,15], and lambs can recover after several weeks [16]. Previous research suggests that early-life nutritional intervention determines the initial rumen microbial community, but the persistence of the effects in later life is weak [17]. Therefore, it has been suggested that alterations of the microbiota for optimizing rumen function may be the most effective approach for rumen development during or immediately following the weaning transition [18]. However, the longitudinal changes in rumen microbiota caused by early weaning are currently unclear.
2. Bacterial Composition at Different Taxonomical Levels
A total of 16 phyla of the rumen contents in both conventional weaning (CON) and early weaning (EW) groups were identified. The main phyla across all samples were Firmicutes with the highest relative abundance, followed by Bacteroidetes, Actinobacteria, and Proteobacteria, accounting for over 95% of total reads (Figure 2A). At 26 d, the relative abundance of Firmicutes in the CON group was 34.8%, while its relative abundance in the EW group increased to 50.8%. At the same time, the relative abundance of Bacteroides in EW was 39.3% compared to its relative abundance in CON with 52.3%. Additionally, Firmicutes in both CON and EW groups increased from 26 to 35 d, and subsequently decreased at 63 d. However, the opposite pattern of Bacteroides with age was observed.
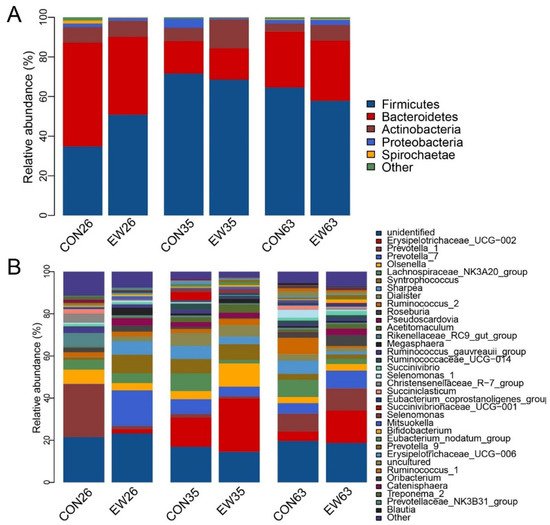
Figure 2. The composition of rumen microbiome at phylum and genus level. (A): The composition of rumen microbiome at phylum level; (B): The composition of major rumen genera. CON26 = control at 26 d; EW = early weaning group at 26 d. The rest can be deduced by analogy.
3. Bacterial Biomarkers for Early Weaning (EW) at Different Ages
To identify the bacterial taxa differentiating CON and EW groups at different ages, Linear discriminant analysis Effect Size (LEfSe) analysis was performed at the genus level. At 26 d, the bacteria with significant differences in the CON group were Prevotella 1, Rikenellaceae RC9 gut group, and Christensenellaceae R 7 group, while the bacterial biomarkers for the EW group were Prevotella 7, Syntrophococcus, Sharpea, Megaspheaera, Pseudoscardovia, and Erysipelotrichaceae UCG-002 (Figure 3A). Upon sampling rumen microbiota at 35 d, Lachnospiraceae_NK3A20_group was enriched in the CON group, but Erysipelotrichaceae UCG-002 was identified as biomarker for the EW group (Figure 3B). At the end of this trial (63 d), rumen microbiota in lambs of the CON group had high relative abundances of Ruminococcus 2 and Lachnospiraceae_NK3A20_group, whereas Erysipelotrichaceae UCG-002 and Eubacterium ruminantium group were over-represented in the EW group (Figure 3C).
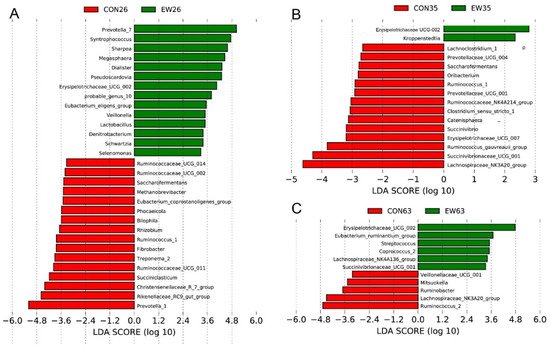
Figure 3. LEfSe analyses of rumen microbiota. LEfSe identified significantly different bacteria at the genus level as differentiating the two groups (control (CON) and early weaned (EW)) at 26 d (A), 35 d (B) and 63 d (C). Genera in this graph were statistically significant (p < 0.05) and had an LDA Score > 2.5, which was considered a significant effect size.
4. Rumen Bacteria Associated with Fermentation Parameters
Briefly, the concentration of Total VFA, molar proportion of acetate and propionate in the EW group increased at 5 days after early weaning. A number of significant correlations were found between the rumen microbiota and fermentation parameters (Figure 5). For example, rumen ammonia concentration was positively correlated with the relative abundances of Erysipelotrichaceae_UCG-002, Acetitomaculum, and Ruminococcaceae_UCG-014; acetate had a positive correlation with Pseudoscardovia; propionate was negatively correlated with the Rikenellaceae_RC9_gut_group but positively correlated with Megasphaera and Sharpea; butyrate was negatively correlated with Pseudoscardovia and Megasphaera.
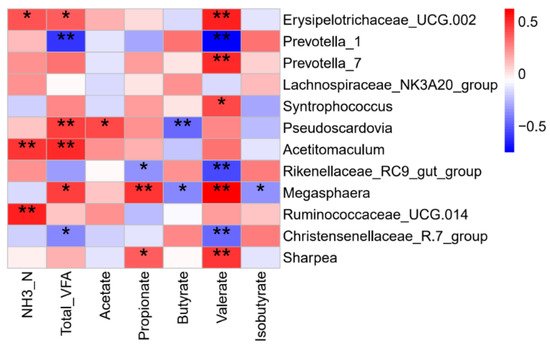
Figure 5. Spearman correlation analysis of ruminal microbiota and rumen fermentation parameters. Color represents the correlation coefficient, with red representing a positive correlation and blue denoting a negative correlation. * p < 0.05 and ** p < 0.01.
5. The Diversity and Richness of Rumen Microbiota Influenced by Early Weaning
The diversity and richness index are usually used to evaluate the stability of an ecosystem. The results show that early weaning caused the decreased richness and diversity of rumen microbiota of lambs, and its impacts mainly happened on 5 and 14 days after weaning at 21 d. At the same time, a significant difference was observed in beta diversity of the rumen microbiota between the EW and CON lambs on 26 d. The dissimilarity of the structure of the rumen microbiota between the two groups decreased on 42 days after early weaning. These results suggest that early weaning changed the process of colonization of the rumen microbiota of lambs in a short period, while the diversity and structure of rumen microbial communities could recover at 42 days after weaning. These results partially differ from previous research which found that the alpha diversity and beta diversity between weaning and post-weaning for lambs receiving the strategy of artificial feeding with milk replacer and weaning at 30 d were not significantly different when compared to controls [2]. Another study of the same feeding strategy showed that alpha diversity was not significantly influenced between pre- and post-weaning whether or not lambs were weaned at 21 or 35 d [13]. Differences with reported results may be related to different feeding strategies. One of the studies pointed out that supplementary feeding with alfalfa before weaning could avoid changes in the rumen microbial diversity and abundance before and after weaning [8]. Feeding mode is an important driver of early microbial colonization, influencing the structure and function of lambs’ gut microbiome [6].
After the birth of young ruminants, the rumen microbiota is in a process of constant change with age and the maturation of rumen [24,25]. In the CON group, the diversity and richness of bacterial communities increased with age, which is in accordance with the results of previous studies [7,11,25]. This is mainly related to the continuous changes in lambs’ breast milk and starter intake to continuously adjust and adapt to rumen fermentation substrates. In EW group, the rumen microbial diversity and richness of lambs at 63 d were higher than at 26 and 35 d, which is consistent with the previous research reported [2,11]. The results indicate that early nutritional intervention could affect the initial microbial formation in the rumen, and the influence of weaning on the rumen community is the most obvious [27]. Early weaning stress has adverse effects on lambs’ growth and health [14], and impacts the richness and diversity of rumen microbiota.
6. The Compositions of Rumen Microbiota Are Associated with Early Weaning
At the phylum level, the rumen microbiota of lambs in control and early weaning groups are mainly Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, which is consistent with previous research results in lambs [8,13,24]. This study shows that early weaning affected the composition of microbes in the rumen of lambs in the short-term. As in the phylum, after early weaning, the relative abundance of Firmicutes in the rumen significantly increased, while the percent of Bacteroides significantly decreased on day 26 of age. After early weaning, solid feed was increased significantly. The increases in relative abundance of Firmicutes are related to high grain feeding, energy harvesting or feed efficiency [28,29,30]. Studies on dairy cows have shown that high grain feeding increased the relative abundance of Firmicutes [30]. However, one study showed no significant difference in relative abundance of any of the top four bacterial phyla between different ages [2] and this may be related to different feeding strategies or weaning age.
7. The Temporal Dynamics of Rumen Microbiota after Early Weaning
Compared with weaning, there are higher numbers of bacterial genera which changed with ages. Relative abundance of Syntrophococcus was lower in EW lambs at 26 d, but retained similar relative abundance with controls at other ages. Syntrophococcus is involved in the utilization of non-fibrous carbohydrates and produces acetic acid [46]. After early weaning, the percent of Syntrophococcus increased, which is similar to the results of the recent study on calves [47]. The relative abundance of Syntrophococcus decreased at 63 d in both groups compared to 35 d, which may be due to ages and diet. Megasphaera is a lactic acid-utilizing bacterium. The proportion of Megasphaera in the early weaning group is higher than that of the control group at 26 and 35 d, which proves its relationship with early weaning and starter intake. Research showed that Megasphaera is an ecologically important rumen bacterium that metabolizes lactate and relieves rumen acidosis induced by a high-grain-diet [48].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10010144
This entry is offline, you can click here to edit this entry!
