Photoluminescence is an especially important and useful mechanism for in situ investigations in tissue engineering, surgery, tissue restoration. Labeling with the aid of organic fluorescent molecules has been popular in clinical trials for years. In recent times, many inorganic components, even nanoparticles, have been proposed to be such candidates. Nonetheless, the toxicity of such particles represents a challenge to practical application because of their composition and nano-size character. A luminescent material with high biocompatibility is a perfect candidate for implantation and clinical application.
1. Hydroxyapatite Doped with Terbium
Terbium is an essential element of the rare earth family that presents complex optical, magnetic, and electronic properties. It is also an appropriate candidate for applications in the glass industry
[1][2], information industry
[3], polymers
[4][5], biochemical sensors
[6][7][8], and solar cells
[9][10][11]. Terbium is unavoidably present in the environment, organisms, and food chains due to its extensive development and utilization. It is necessary for the human body and/or environment to determine and monitor exposure to Tb
3+ because of its increased discharge of toxic properties and other adverse effects
[3].
Li et al.
[12] proposed an appropriate approach to synthesize nano-hydroxyapatite, whose luminescent properties could be stimulated under visible excitation. The method replaces the surface calcium ions on hydroxyapatite nanoparticles with a specific quantity of Tb
3+. They prepared an inorganic biological probe by doping 20 nm hydroxyapatite with terbium ions. The calcium ions on the hydroxyapatite particle’s surface can be partially substituted by terbium. Nevertheless, an extremely low amount of the doped Tb
3+ ions on the hydroxyapatite surface can increase its luminescence, maintaining, at the same time, the principal physicochemical properties and bioactivity of hydroxyapatite. The nanoparticles of the terbium-doped hydroxyapatite were obtained at room temperature, and the atomic ratio of Tb:(Ca + Tb) was 2:100. It was proved experimentally that the Tb-hydroxyapatite nanoparticles could induce a constant luminescence. Moreover, these fluorescent Tb-HA nanoparticles can be incorporated by living cells. Even if the total number of Tb
3+ ions in the material is moderate, it should be noted that the terbium is nontoxic, because its lethal dose is 50 g via an oral route (LD50PO), which is more than 5 g/kg of terbium nitrate in rats. Using a visible light beam (488 nm), the green emission of the hydroxyapatite nanoparticles could be stimulated. The ready internalization of the nanoparticles and its steady fluorescence indicates that the terbium ion-doped hydroxyapatite represents an excellent inorganic probe for living cells with high biocompatibility. After incubation with rabbit bone marrow mesenchymal stem cells (MSCs) in culture, the luminescent hydroxyapatite was clearly visible under a fluorescent microscope. It was determined that the obtained terbium-doped hydroxyapatite could represent a promising biological probe for cellular research. These results indicate that the Tb-doped HA has enormous potential to advance the development of luminescent nanoparticles, which is an important subject in nanobiotechnology and medicine. Moreover, to evaluate the potential applications of HA-Tb
3+ in the biomedical field, studies on the biological reaction of MC3T3-E1 cells with different concentrations of Tb
3+-doped hydroxyapatite (HA-Tb) nanorods (25, 50, and 100 μg/mL) were performed
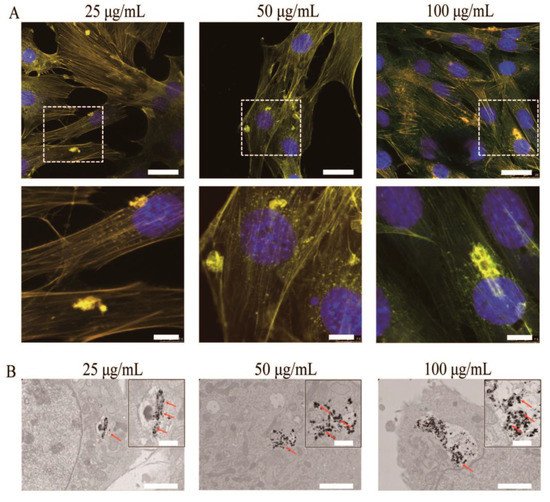
[13]. The photoluminescence of the internalized hydroxyapatite in the intracellular cytoplasm was visible under a fluorescent microscope, and the cells retained their natural morphology (
Figure 7).
Figure 7. (A) Representative fluorescent images of MC3T3-E1 cells after incubation with HA-Tb nanorods for 24 h at 37 °C. (B) Transmission electron microscopy images of MC3T3-E1 cells after incubation with HA-Tb nanorods.
2. Hydroxyapatite Doped with Erbium
The existence of erbium (Er
3+) in rib bones has been studied, and it is supposed that the doping of hydroxyapatite with erbium ions could improve the biological properties of hydroxyapatite. Er
2O
3 may be exploited to dope calcium phosphate with Er
3+ ions to stipulate photo sites for absorption laser energy, which may contribute to the densification of the manufactured enamel mineral over typical dentine. Moreover, the photoluminescent properties of Er
3+ can be used to study the in vivo resorption of Er-HA, which can be applied to supervise the bone healing process
[14].
Alshemary et al.
[14] prepared nano-sized erbium-doped hydroxyapatite using a microwave-assisted precipitation method. It was observed that the crystallinity and the particle dimensions reduced from 75% and 78 nm to 36% and 25 nm, respectively, with the growth of the Er
3+ amount for doping hydroxyapatite. The photoluminescence test revealed the existence of red and green emission in the spectra. The study showed that Er-HA could be a promising alternative as a sensing material in the future. In vitro bioactivity tests for erbium-doped hydroxyapatite materials conducted in simulated body fluid (SBF) revealed the production of an apatite layer with a (Ca + Er)/P ratio in the range of 1.59–1.72. For that reason, Er-HA can be designed as possible biomedical material. Alshemary et al.
[14] also described a promising method to obtain mesoporous erbium-doped hydroxyapatite by applying a microwave-assisted wet precipitation approach. They obtained results that showed the production of crystalline Er-HA with a crystallite size of 25 nm and spherical and rod-like morphology. The transmission electron microscopy (TEM) analysis proved the mesoporous structure of the obtained particles.
The optical spectra of erbium-doped HA consist of seven electron transitions, although a blue shift in the energy bandgap (Eg) was noticed upon the increase of the Er
3+ amount. The photoluminescence spectra consisted of green and red emissions
[14]. An in vitro bioactivity test performed in simulated body fluid (SBF) showed that the substitution of Ca
2+ with Er
3+ ions into the hydroxyapatite structure leads to a rapid discharge of Er
3+ ions, showing a strong increase of apatite grains on the surface of the Er-HA pellets with a Ca/P ratio of 1.72
[14].
Pham et al.
[15] synthesized erbium-doped hydroxyapatite using a coprecipitation method. The near-infrared luminescence emission of hydroxyapatite may be obtained by doping with erbium. The photoluminescent properties of the erbium-doped hydroxyapatite utilizing a thermal annealing treatment (=1100–1200 °C) were demonstrated by a strong band emission at ~1540 nm, which was more intense than those of the lower-temperature-annealed erbium-doped hydroxyapatite. This improvement of the photoluminescence was associated with the incorporated β-TCP (tricalcium phosphate) phase in the material’s microstructure, which is an inhibitor of photo quenching. Erbium-doped hydroxyapatite can be used as a potential material in applications such as waveguide telecommunication and biomedicine
[15].
Erbium represents an appropriate element for doping the materials designed for luminescent and biocompatible materials because of its light emission spectra and great biocompatibility
[15].
3. Hydroxyapatite Doped with Europium
Lanthanide ions, such as europium or terbium, present regular fluorescence for cell imaging under a specific excitation wavelength. Particularly, doping with europium, which has 4f–4f intraorbital electronic transitions covering the visible and near-infrared ranges leads to relevant material for biomedical applications in the near-infrared spectral range
[16].
Escudero
[17] synthesized europium-doped calcium hydroxyapatite nanoparticles using a microwave-assisted approach. An aqueous solution with a stoichiometric amount of sodium phosphate monobasic was combined with another aqueous solution consisting of calcium nitrate tetrahydrate, europium-(III) nitrate pentahydrate, and polyacrylic acid. The final concentration of calcium, phosphate, and poly(acrylic acid) were 0.06 mol dm
−3, 0.036 mol dm
−3, and 2 mg cm
−3, respectively. The solution’s pH was adjusted to 11 by adding aqueous ammonia. Due to their luminescent properties (red luminescence), their small toxicities (negligible toxicity for Vero cells), and the functionalization with poly(acrylic acid), which induces the capacity for further functionalization with molecules of biomedical interest, these nanophosphors are a promising candidate for use as potential tools for biomedical applications.
4. Hydroxyapatite Doped with Lanthanum
La3+-substituted hydroxyapatite displays excellent biocompatibility compared with pure hydroxyapatite. A few studies have been conducted to demonstrate the benefits of lanthanum in the biomedical field.
Mayer et al.
[18] obtained hydroxyapatites by precipitation from an aqueous solution with La
3+ (0–0.75%) and carbonate (0–6.1%) at a controlled pH of 7.0. The assimilation of La
3+ was 90–95% complete. Due to nonstoichiometry, the Ca/P (1.54–1.63) ratios were low. With the aid of IR spectroscopy, it was determined that the carbonate from the samples was a B-type carbonate. The lattice parameters of the hexagonal apatite structure were not changed because of the La
3+ amount. After heating to 800 °C, the noncarbonated samples were partially transformed into β-Ca
3(PO
4)
2. Thermogravimetric analysis indicated a release of 0.4 mol adsorbed and 1 mol crystalline water up to 400 °C and the deterioration of the carbonate up to 900 °C in the obtained samples. These results reveal that La
3+ could replace Ca
2+ in the crystal lattice of hydroxyapatite
[18].
Ghosh et al.
[19] obtained phase pure hydroxyapatite and lanthanum phosphate powders using a wet chemical synthesis method. Composite samples were obtained by mixing the powders, pressing, and sintering. The composite nature of the prepared pellets incorporates up to 50 wt.% lanthanum phosphate (H0 − 100%Hap, HL1 − 90% Hap + 10%LP, HL2 − 80% Hap + 20%LP, HL3 − 70% Hap + 30%LP, HL4 − 600% Hap + 40%LP, HL5 − 50% Hap + 50%LP) was achieved even up to sintering at 1150 °C. A continuous decline in densification, bending strength, and hardness was reached by increasing the content of the lanthanum phosphate. It was found that the obtained composites could be drilled by solid carbide drill bits up to 1150 °C. The composites with a high lanthanum phosphate amount presented better processability compared with the ones with a lower lanthanum phosphate content, and the force and torque required were much higher. The materials sintered at 1200 °C revealed a new phase. Thus, the composite structure was not maintained, and the durability was also not obtained for any amount of the lanthanum phosphate content. All the different prepared compositions (H0, H1, H2, H3, H4, H5) demonstrated positive character for in vitro bioactivity and biocompatibility assay. The cell viability also displayed the irrelevant difference in the results with the increasing lanthanum phosphate amount
[19].
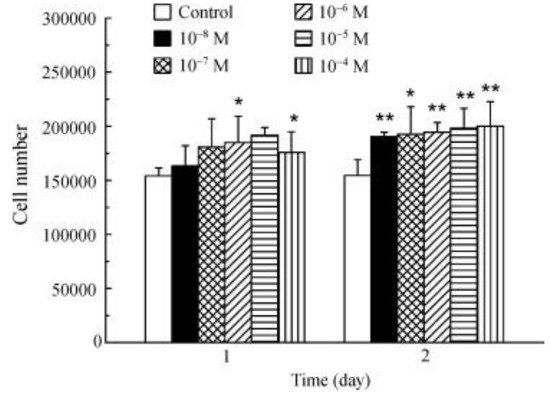
La
3+ promotes osteoblast proliferation (
Figure 13). The incubation of osteoblasts with La
3+ (1 × 10
−8–1 × 10
−4 M) showed that all the tested concentrations significantly increased the cell number after 2 days
[20].
Figure 13. Effect of La
3+ on osteoblasts proliferation (*
p < 0.05; **
p < 0.01)
[20].
Jadalannagari et al.
[21] synthesized rod-shaped nanoparticles of lanthanum-doped hydroxyapatite (La-HA) using a modified sol-gel method at a low temperature of 100 °C. The (La + Ca) :P proportion was maintained at 1.67 while the La/(Ca + La) ratio was varied as
x = 0.02, 0.06, 0.1. By increasing the concentration of La from
x = 0.02 to 0.1, an increase in crystallinity and crystallite size was observed. These obtained particles were internalized by the human embryonic kidney and human adenocarcinoma cells and showed fluorescence when detected under tetramethylrhodamine (TRITC) and fluorescein (FITC) filters using epifluorescence microscopy. Lanthanum-doped hydroxyapatite nanoparticles with
x = 0.02 displayed the highest internalization, maximum intracellular fluorescence, and no cytotoxicity. It was observed that cell viability and internalization decreased with increases in the La amount. Nanoparticles corresponding to
x = 0.1 presented spindle-shaped morphology
[21].
Nano-rods of pure hydroxyapatite and lanthanum-doped hydroxyapatite were obtained by Ahymah
[22] using a sol-gel route and calcium nitrate and diammonium hydrogen phosphate as precursors. The samples did not contain La
2O
3 or β-TCP phases, compared with the samples obtained by the solid-state and co-precipitation methods. The control sample was noted as CHAp, and the lanthanum-doped samples with 10 mM, 20 mM, 30 mM, 40 mM, and 50 mM were denoted as L1HAp, L2HAp, L3HAp, L4Hap, and L5HAp. Enhancement in the amount of lanthanum resulted in an increase of crystallinity and crystallite size. Furthermore, enhancements in the specific surface area (31%) and hardness (14%) were noticed in the doped samples. The tests using phosphate-buffered saline (PBS) showed a reduction in the dissolution of the samples because of the enhancement in the dopant concentration.
An in vitro study of drug release was determined by measuring the absorbance using a UV spectrophotometer at 230 nm. The hydroxyapatite sample and amoxicillin were taken in a 2:1 ratio, and it was mixed thoroughly and made into a pellet. The bactericidal effect against Gram-positive and Gram-negative bacteria showed high antimicrobial-resistant activity of the samples when they were functionalized with amoxicillin
[22]. La
3+-doped hydroxyapatite is a promising approach in the biomedical field for biocompatible fluorescent probes applied in cellular internalization and drug-releasing agents over a prolonged period.
5. Hydroxyapatite Doped with Dysprosium
Dysprosium is a heavy rare earth element (HREE). Dysprosium-165, its radioactive isotope, was studied for potential applications in the field of medicine. Radiation with dysprosium-165 has been demonstrated to be more efficient in treating injured joints than traditional treatment and surgery. Dysprosium-doped hydroxyapatite represents an attractive option for a biocompatible ceramic material for luminescence imaging applications.
Tesch et al.
[23] prepared luminescent and magnetic hydroxyapatites by doping with europium (Eu
3+) and dysprosium (Dy
3+). A co-doping of Eu
3+ and Dy
3+ was used to blend the requested physical properties. Both REE ions, Eu
3+ and Dy
3+, were assimilated into the hydroxyapatite crystal lattice, where they preferentially chose calcium sites. Eu-doped hydroxyapatite presents a dopant concentration with persistent photoluminescent properties, while Dy-doped hydroxyapatite exhibits paramagnetic comportment because of the high magnetic moment of Dy
3+. Co-doped HA nanoparticles blend both properties into one single crystal. Curiously, the multimodal co-doped hydroxyapatite increased its photoluminescent properties due to the energy transfer from the Dy
3+ sensitizer to the Eu
3+ activator ions. Eu:Dy:HA presents strong transverse relaxation effects with maximum transverse relativity of 83.3 L/(mmol·s). Because of their tunable, photoluminescent, magnetic properties and cytocompatibility Eu-HA, Dy:HA, and Eu:Dy:HA serve as alternative biocompatible ceramic materials with applications in luminescence imaging and may also be suitable as a contrast agent for magnetic resonance imaging (MRI) in implantology or functional coatings
[23].
This entry is adapted from the peer-reviewed paper 10.3390/nano9020239