Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Electrical & Electronic
Electrochemical devices enable the measurement of a wide variety of ions, such as hydrogen ions, electrolytes, heavy metals, and nutrients.
- electrochemical devices
- chemical sensors
- ionic detection
1. Introduction
The charged ionic species, such as electrolytes (Na+, K+, Cl−) and heavy metals (Pb2+, Cu2+, Cd2+, Zn2+, Hg2+), play key roles in physiological processes for biological developments, including neural communication networks and biofeedback systems for cardiovascular regulation [1,2,3,4,5,6,7,8,9]. These charged species regulate the pH level in water ecosystems and help in plant growth for the environment [5]. However, disproportionate concentration of ionic species can be toxic for many biological processes [1,2,3,5,7]. As an example, heavy metal concentration in the human body is associated with a higher risk for Alzheimer’s disease [2]. High concentrations of these heavy metal species are often caused by pollution from modern factories, as well as hazardous substances from the developments of agriculture [1,2,3]. Toxic ionic species can also be absorbed into the body. Mercury substances are found in food sources involving aquatic species and in day-to-day life [2]. The ions from industrial waste and old piping can make their way into food and water sources [4]. Therefore, the potential value of continuous monitoring and tracking down ionic analytes are significant. In preventive healthcare, it is essential to develop ion-sensitive devices that can track and monitor the ionic concentration levels that have important implications on the health of an individual.
Electrochemical devices enable the measurement of a wide variety of ions, such as hydrogen ions, electrolytes, heavy metals, and nutrients [5,6,7,8]. For instance, one of the common methods to measure pH is using glass electrodes. Figure 1 shows various usage of ion-selective devices. In recent years, they are extensively used in healthcare, environmental monitoring, food quality assessment, agriculture cultivation, and drug tests [2,5,7,9]. These electrochemical devices can be fabricated as electrochemical sensors through roll-to-roll (R2R) printing, physical vapor deposition (PVD), and semiconductor compatible processes [9]. They are capable of multiplex sensing, which provides users a pathway toward comprehensive analyses.
Figure 1. Wide application of sensors for ionic analytes.
Developing cost-effective and high-throughput methods of fabricating sensing components are critical for the commercialization of one-time throw electrochemical devices. In various available printing techniques, such as R2R gravure printing, large-scale production of low-cost and robust devices can become possible. The simple printing mechanism of R2R gravure allows faster printing speeds and also provides superior resolution, consistency over the screen, offset, and flexography printing techniques [9,10]. Due to their promising usage, the advancement of integrated electrochemical devices is fueled by rapidly growing research. They consist of an ionized functional layer, such as film or membrane, that is selectively responsive to targeted ions and electrochemical transducers to relay chemical signals to electrical signals when target analyte ions are in contact with the layer. The commonly used electrochemical sensing technologies are based on potentiometric, voltammetric, capacitance, and impedance measurements [11,12,13,14,15,16,17,18,19]. Heavy metals are important indicators of the toxicity of an environment and human health condition. Human body fluids (such as blood, sweat, and urine) contain various metabolites, electrolytes, proteins, and heavy metals. Research has shown that various heavy metals can be found in human body fluids, which are closely related to human health status [9,11]. For instance, high Cu2+ accumulation in the human body can lead to Wilson’s disease [4]. Additionally, Pb2+ and Hg+ are toxic substances on human body systems, including cardiovascular, immunological, and nervous systems [20,21,22,23,24,25]. Some studies are developing electrochemical sensors based on organic conducting polymers, such as composites with carbon nanotubes (CNTs), to detect trace heavy metal ions in water [26]. CNTs have good properties such as chemical, mechanical, electrical, and environmental stability. Graphene-based materials are promising due to their low electronic noise and zero bandgap. They have high electron mobilities which are essential for heavy metal detection [27,28]. Organic conducting polymers (OCPs), such as polypyrrole, polyphenylene, polyaniline, polyacetylene, polythiophene, etc., have advanced electrochemical properties toward metal ions detection [29]. Because of their collective redox properties, OCPs have good sensitivity to minor electrochemical perturbations [30].
2. Integration of Electrochemical Sensors for Ionic Analytes and Energy Harvesting Electronics
Electrochemical sensors for ionic analytes are powerful devices because of their portability. With the development of various mobile electronics, technology that is capable of supplying a battery in environments where it is not possible to provide a stable power supply is essential. In this regard, energy harvesting has attracted attention and energy harvester shows a viable method to overcome the limitation of battery capacity that can constrain the long-term operation of implantable and wearable devices [104,105,106,107,108]. These energy harvesting sensors are self-powered from the ambiance, and the converted electric power can be applied in the sensing process instantly. They do not need an extra power source, time-consuming charging duration, and complex procedures for sensing. Therefore, energy harvesting is an attractive functionality to include with a wearable device for ionic analytes [104,108]. A self-powered device will be advantageous to those who need continuous monitoring for fitness management and health monitoring. In the following section, we are going to introduce several mechanisms of self-powered energy harvesters integrated with an electrochemical device for ionic sensing.
2.1. Triboelectric-Powered Energy Harvester for Electrochemical Ionic Sensing
Despite much research on wearable energy harvesters, most of them suffer from poor robustness, complex fabrication process, and low power density. The above issues make them inconvenient for continuous monitoring. In triboelectric-powered energy, triboelectric is a static electricity on which certain materials become electrically charged after they are separated from the original material with which they were in contact. This is an effective energy harvesting technique, which provides necessary power for small electronics.
In order to generate a sustainable and energy-efficient wearable device, Yu Song et al. [104] proposed a wearable freestanding triboelectric nanogenerator (FTENG) energy harvester based on a flexible printed circuit board. This device efficiently allows the collection of power from human motion with battery-free, robust, and mass-producible properties. The engineered FTENG demonstrates a triboelectric and battery-free system to power multiplexed sweat sensing and wireless data transmission during on-body human exercise trials. In this work, sensitivities are measured with physiologically relevant Na+ concentrations (12.5 to 200 mM) and pH levels (4 to 8). Sensitivities of Na+ and H+ are 58.63 and 56.28 mV per decade concentration, respectively. The result is close to Nernstian sensitivities and high sensing performance is proven. Figure 4A shows a battery-free FTENG-powered wearable sweat sensor system (FWS3) for wireless and non-invasive sweat monitoring. The left side shows integrated human motion energy harvesting FWS3, which has power supply to Bluetooth-based wireless data transmission in real-time for health status monitoring. This device includes a mobile user interface and microfluidic-based sweat sensing functionality. The right side shows different working frequencies of FTENG and its current output during the energy harvesting process.
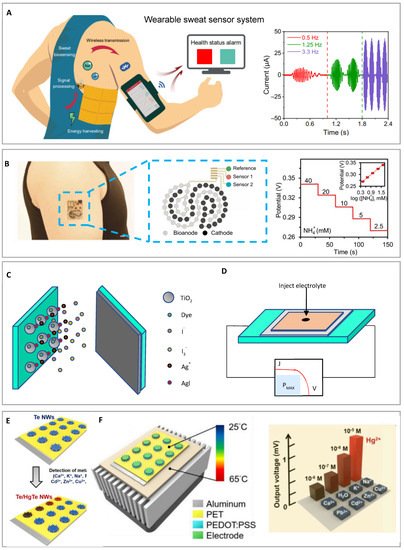
Figure 4. (A) From left to right shows integrated human motion energy harvesting a freestanding triboelectric nanogenerator (FTENG)-powered wearable sweat sensor system and the output energy. Reproduced with permission. Copyright 2020, American Association for the Advancement of Science [104]. (B) From left to right shows a biofuel-powered energy harvester and its output energy. The potentiometric responses of the NH4+ sensors are measured in 40 to 2.5 mM NH4+ solutions and they demonstrate near-Nernstian sensitivities of 60.3 mV per decade of concentration. Reproduced with permission. Copyright 2020, American Association for the Advancement of Science [108]. (C) Shows dye-sensitized solar cell (DSSC), which will produce a change with its electrical characteristics in response to the ionic species, while introducing Ag ions into the electrolyte of a DSSC results in the adsorption of Ag ions as well as the formation of metallic precipitation on the mesoporous TiO2 electrode. (D) Shows a representation of the testing procedure. The devices were connected to a Solartron Analytical Modulab test system. The impedance spectra and electrical characteristics were measured under 1 sun illumination and in the dark. (C,D) Reproduced with permission. Copyright 2014, Royal Society of Chemistry [116]. (E) Shows self-powered thermoelectric nanosensor and sensing process for Hg2+ detection. Tellurium nanowires (Te NWs) here acted as a thermoelectric material as well as a sensor. (F) The electrolyte is sucked into the vacuum cavity, and its sensing process for mercury ions are reported after electrochemical reaction. Finally, the electric output was performed by using Peltier system to create a temperature gradient across the thermoelectric nanosensors. (E,F) Reproduced with permission. Copyright 2019, Elsevier [119].
2.2. Biofuel-Powered Energy Harvester for Electrochemical Ionic Sensing
Due to interests in alternative fuels in the past few years, biofuel cells have become an important topic of research [105,106,107,108]. This presents a unique opportunity to integrate with electrochemical sensors for ionic sensing. A fuel cell is an electrochemical cell that can generate current from reactions between the chemical species flowing into the cell [105]. When biofuel cells can be a power source for ionic-sensing wearable sensors, it provides a way for long-term biosensing on a standalone platform, where the energy generated from sweat can be used in ionic sensing [106].
Hee Uk Lee et al. [107] developed a graphite oxide/cobalt, hydroxide/chitosan composite material, which has high specific surface area and redox activity, and the maximum power density is 517 μW·cm−2. You Yu et al. [108] presented a biofuel-powered energy harvester on skin sensing platforms. Many existing sensing platforms of electronic skin rely on batteries or near-field communication for power charging, which is inconvenient and restricted by the locations of the wireless equipment. This work overcame the above limitation and demonstrated a battery free biomedical device with fully perspiration-powered electronic skin (PPES). The key metabolic biomarkers such as NH4+, glucose, urea, and pH are monitored and transmitted wirelessly in a continuous way to a mobile device via Bluetooth low energy (BLE). Figure 4B shows photographs of a PPES on a testing subject’s arm, a reference sensing electrode, and an ion sensor on a flexible BFC-biosensor patch. The potentiometric response of the NH4+ is measured under 2.5 to 40 mM NH4+ solutions. The outcome shows a linear potential output versus logarithmic concentrations of the target analyte, with near-Nernstian sensitivities of 60.3 mV per decade of concentration for NH4+ sensors. This work demonstrates PPES that harvests energy from human lactate biofuel cells through human sweat.
2.3. Photovoltaic- and Thermoelectric-Powered Energy Harvester for Electrochemical Ionic Sensing
Solar energy is one kind of renewable energy source, with its promise to supply a share of the global energy demand [109,110]. In particular, photovoltaic (PV) devices are used to generate electrical power via semiconductors that exhibit photovoltaic effects to convert solar radiation into direct current. Several PV technologies, such as perovskite solar cells and dye-sensitized solar cells (DSSC), were reported. PV have high potential for mass production with manufacturing methods such as screen printing, inkjet printing, or slot-die coating to produce electrochemical sensors for ions [111,112,113,114]. In prior demonstrations of the concept, a dye-sensitized solar cell is used for both sensitive detection and power generation of ionic analytes, which kick off a new pathway for integration and ultra-miniaturization. Since its beginning, dye-sensitized solar cell technology has proven stability and simplicity of fabrication while being able to sustain competitive efficiencies of power conversion [115].
A versatile detection strategy was demonstrated by Kanika L. Agrawala and Max Shtein [116]. The proposed device of this work was for sensing, and the power generation was achieved using a single DSSC device. A DSSC is responsive to the occurrence of ionic species, such as silver, and that generates a change in its electrical characteristics. When introducing silver ions into the electrolyte of a general operating DSSC, the Ag ions will be adsorbed, and the metal will be deposited on the mesoporous TiO2 electrode. Then, it reduces the driving level for charge injection while the deposited metals can make dye sites inactive and halt the recombination between electrons. This approach can reliably detect microscale concentrations of charged species in solutions without making substantial adjustments to a well-understood photo-electrochemical system. The DSSC technology for autonomous sensing of metal ion pollution in environments with high sensitivity and repeatability can pave the path for new applications. Figure 4C shows dye-sensitized photovoltaic cell and the response of the presence of ionic species that produces a change in its electrical features. The Ag ions are introduced into the electrolyte of a DSSC under operation mode, and the adsorption of Ag ions form metallic precipitations on the TiO2 electrode. Figure 4D shows the method for testing the sensor through injecting electrolytes.
Thermoelectric (TE) devices have received attention since they are able to convert temperature gradients into electricity. They possess the potential to become a candidate that allows for the operation of self-powered sensors that are separated from a localized power supply [117,118]. Yu-HsiangTsao et al. [119] showed that the mechanism of thermoelectric generators can be implemented to design a modern prototype of a self-powered sensor for the detection of Hg2+ ions. Generally, the performance of thermoelectric generators depends on the critical thermoelectric materials, which is the key factor to determine the sensing capability of the thermoelectric sensor. For the past few years, nanostructures of tellurium have been proven to be highly efficient thermoelectric materials. Tellurium shows novel properties and has the ability to be applied in piezoelectric nanogenerators [120,121]. Here, tellurium nanowires (Te NWs) were selected as the essential material for the self-powered thermoelectric sensor. Their excellent thermoelectric performance property comes from HgTe nanocrystals, where the specific reaction of Hg2+ ions and Te NWs leads to higher thermoelectric efficiency. The as-constructed thermoelectric sensor has high selectivity and sensitivity (detection limit of 1.7 nM) for Hg2+ ion.
The proposed thermoelectric sensor can supply an intense electric output signal even when it is driven by a small temperature difference. Figure 4E shows the structure of the self-powered thermoelectric nanosensor and its sensing process for the detection of mercury ions vs. different metal ions. Figure 4F shows the output measurement of multiple ions. After 30 min of reaction time, PEDOT:PSS is coated on the wells to finalize the fabrication of thermoelectric nanosensors. Finally, electrical signal can be outputted across the thermoelectric nanosensors.
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors10010022
This entry is offline, you can click here to edit this entry!
