Phosphatidylinositol 4,5- bisphosphate (PI(4,5)P2) is a minor but ubiquitous component of the inner leaflet of the plasma membrane of eukaryotic cells. However, due to its particular complex biophysical properties, it stands out from its neighboring lipids as one of the most important regulators of membrane-associated signaling events. Despite its very low steady-state concentration, PI(4,5)P2 is able to engage in a multitude of simultaneous cellular functions that are temporally and spatially regulated through the presence of localized transient pools of PI(4,5)P2 in the membrane.
- phosphoinositides
- lipid domains
- calcium-induced clustering
1. Introduction
PI(4,5)P2 is a member of the phosphoinositide (PI) lipid family. PIs are a small group of glycerophospholipids derived from phosphatidylinositol. These lipids consist of a characteristic inositol headgroup, which can undergo reversible phosphorylation and dephosphorylation, leading to the formation of seven distinct phosphorylated species. While the parent lipid phosphatidylinositol represents roughly 10% of total membrane phospholipids in the eukaryotic cell, the phosphorylated derivatives account only for around 2–3% [1], with PI(4)P and PI(4,5)P2 representing the bulk of these lipids [17]. Each of these seven species has a distinct subcellular distribution with predominant localization in subsets of membranes. Additionally, within a given membrane the localization of a specific PI can be heterogeneous. Many PIs are overall in low abundance in the membrane but they can be found at high local concentrations in membrane domains not readily detected by conventional techniques [3,17]. For a historical review on inositol lipids, see Irvine (2016) [18]. Over the last couple of decades, PIs have been found to be one of the most ubiquitous signaling entities in eukaryotic metabolism. Their reach extends from controlling organelle biology to regulating cellular growth. Due to this all-encompassing reach, they have also been linked to a number of human diseases. In fact, the inositide signaling pathway is considered a promising pharmaceutical target. For an excellent review on the major developments on PI cellular biology and their impact on disease, see Balla (2013) [3].
2. Lateral Organization of PI(4,5)P2
PI(4,5)P2 lateral organization in cells has been studied through a variety of techniques from fluorescence correlation spectroscopy (FCS) and fluorescence recovery after photobleaching (FRAP) to atomic force microscopy (AFM). In FCS experiments carried out in Rat1 fibroblasts and HEK cells, researchers microinjected micelles of fluorescent labelled-PI(4,5)P2 into cells and showed that the diffusion coefficient of PI(4,5)P2 in these cells is significantly lower than expected for free phospholipids. The simplest interpretation of this result is that approximately two-thirds of PI(4,5)P2 in the inner leaflet of the plasma membrane is somehow sequestered [44]. Studies in PC12 cells have also shown, using Stimulated emission depletion (STED) microscopy [45] and Stochastic optical reconstruction microscopy (STORM) imaging techniques [46], that PI(4,5)P2 is highly enriched in nanometer-sized membrane domains, specific to this cellular model.
In fact, while the presence of segregated PI(4,5)P2 pools can be partly explained by localized PI(4,5)P2 synthesis and degradation through several kinases and phosphatases [47], it is also evident that membrane diffusion rates, in the absence of significant obstacles for diffusion, will always be higher than concentration changes due to enzymatic activity causing PI(4,5)P2 to diffuse away faster than it can be produced. This means that it is unlikely that local synthesis can result in significant changes in the submicroscopic organization of PI(4,5)P2 in the membrane [43]. PI(4,5)P2 interactions with other cellular binding partners could alternatively explain the observed lateral organization of this phosphoinositide. Interactions with proteins, divalent cations, cholesterol, and the cytoskeleton are the ones most likely to have such an impact. In this review, we will give particular attention to the often neglected effect of divalent cations on the lateral organization of PI(4,5)P2.
2.1. Sequestration by Proteins
One way to explain PI(4,5)P2 lateral organization in the plasma membrane of cells is that proteins can act as reversible buffers, binding much of the PI(4,5)P2 present and then releasing it locally in response to specific signals [48]. Theoretical simulations predict that such sequestration can be achieved not only through specific interactions with PI(4,5)P2 but also through nonspecific electrostatic interactions. In fact, polybasic proteins are able to sequester a lipid with a valence of ~4 (such as PI(4,5)P2) 1000-fold more effectively than a lipid with a valence of ~1 (such as PS) [49,50]. Due to its highly negatively charged headgroup, PI(4,5)P2 was confirmed to interact strongly with polybasic stretches of amino acid residues [43,51]. Through these polybasic stretches, several proteins were found to laterally sequester PI(4,5)P2 molecules in a reversible manner [52,53]. For an efficient buffering of PI(4,5)P2 levels, these proteins would have to be present at a concentration comparable to PI(4,5)P2, localize to the plasma membrane and be able to bind PI(4,5)P2 with high affinity while being able to release it in response to stimuli. Proteins such as myristoylated alanine-rich C-kinase substrate (MARCKS) [50,53,54], Growth Associated Protein 43 (GAP43) [48,55], CAP23[48], among many others, have been shown to be able to sequester PI(4,5)P2 in such a manner. In the case described above of PI(4,5)P2 domains detected in PC12 cells, these were found to be associated with the sequestration of PI(4,5)P2 to clusters of the SNARE protein syntaxin-1 [45,56,57]. This sequestration by syntaxin-1 is critical for the regulation of SNARE-dependent membrane fusion [58,59].
Employing fluorescence and electron paramagnetic resonance spectroscopic tools, McLaughlin, Cafiso, and co-workers [50,52] showed that a 24 aa peptide corresponding to the effector domain of MARCKS was able to efficiently sequester an average of 3 PI(4,5)P2 molecules through non-specific electrostatic interactions. Importantly, this sequestration occurred even in the presence of physiological concentrations of the monovalent acidic phospholipid PS, confirming theoretical predictions. MARCKS sequestration of PI(4,5)P2 has been shown to be important in the PI(4,5)P2 mediated activation of TRPC-family Ca2+ channels [60], in the endocytosis of the amyloid precursor protein (APP) [61], and in the synaptic clustering of PI(4,5)P2 [62].
2.2. PI(4,5)P2 Interactions with Divalent Cations
Several studies have shown that PIs and PI(4,5)P2, in particular, are able to establish strong electrostatic interactions between their negatively charged headgroups and divalent cations. In the cellular PI(4,5)P2 context, calcium and magnesium stand out. Calcium is a common player in signal transduction and a second messenger in cells. Its levels are strictly controlled and maintained at low levels in the cytosol, with normal intracellular levels at around 100 nM (20,000 fold lower than extracellular levels) [63]. Upon stimulation, however, several signal transduction pathways can lead to transient increases of intracellular calcium concentration up to around 1 μM, with local concentrations in the vicinity of open calcium channels reaching hundreds of μM, before being regulated back to normal levels [64]. In fact, PI(4,5)P2 has been reported to be associated with a variety of Ca2+ channels and a great number of these require PI(4,5)P2 for proper function [3]. Magnesium, on the other hand, is a less studied modulator of cell function. Magnesium levels are well buffered in a narrow millimolar range between 0.25 mM and 1 mM [65,66] and are thus kept at a much higher concentration than those of calcium. Both divalent cations have been shown to bind strongly to PI(4,5)P2 and influence its lateral organization dramatically as discussed below.
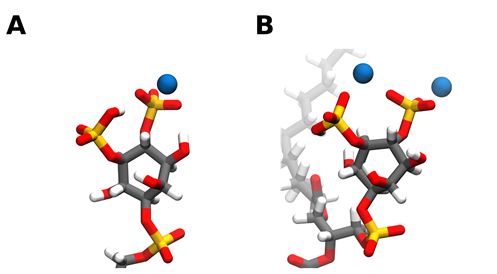
Through hybrid Quantum mechanics/molecular mechanics (QM/MM) experiments we can get an insight on the molecular basis for cation binding to PI(4,5)P2 [31]. From a molecular point of view, when binding to a single PI(4,5)P2 lipid, both calcium and magnesium bind to PI(4,5)P2 either in between the phosphomonoester groups (Figure 1B) or solely near the 4-phophomonoester (Figure 1A). However, simultaneous binding between the two phosphomonoester groups is approximately 10 kcal/mol more unfavorable [31]. Divalent cation binding to the phosphodiester group has also been observed [67].
When analyzing the free energy associated with the removal of each divalent cation from its binding position, significantly more energy is required to remove calcium into the bulk water than it is for magnesium. The difference in free energy could come from the fact that, in contrast to calcium, magnesium appears to retain its first hydration shell in its equilibrium binding position. This causes its equilibrium binding position to be further away from the headgroup and leads to the formation of fewer hydrogen bonds, on average, between the headgroup and the surrounding water molecules. Interestingly, it was also shown that upon binding to calcium, the remaining PI(4,5)P2 headgroup proton at physiological pH could be favorably displaced and that the effective size of the PI(4,5)P2 headgroup would significantly decrease [31]. In the presence of magnesium, the dissociation of the remaining proton was not favorable, however, the decrease in effective headgroup surface area was also observed albeit to a lesser extent. All of these cation-induced changes can and will affect PI(4,5)P2 dynamics, thus influencing local membrane dynamics as well as its interactions with protein binding partners.
Figure 1. Snapshots of calcium ions interacting with the PI(4,5)P2 headgroup phosphates. Calcium can bind to PI(4,5)P2 either solely near the 4-phophomonoester (A) or in between the phosphomonoester groups (B). However, simultaneous binding between the two phosphomonoester groups is approximately 10 kcal/mol more unfavorable [31]. Carbon atoms are colored in grey, hydrogen in white, oxygen in red, phosphorus in orange, and calcium in blue. Snapshots obtained from a simulation of a bilayer consisting of 95:5 mol ratio POPC: PI(4,5)P2 in the presence of calcium in a 5:1 calcium to PI(4,5)P2 ratio, using the CHARMM36m forcefield run in GROMACS2019. Images were modeled using VMD.
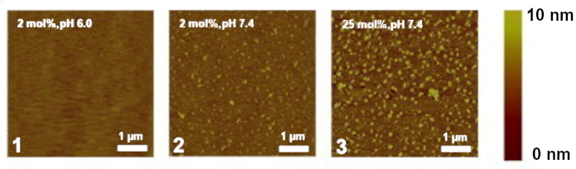
Apart from simply binding to PI(4,5)P2, both divalent cations also have the ability to crosslink PI(4,5)P2 lipids. This induces the formation of very stable cation-induced PI(4,5)P2 nanodomains. It has been shown through different experimental techniques that divalent cations, and especially calcium, are able to induce the formation of PI(4,5)P2 nanodomains, even at physiological concentrations of cation and lipid. In lipid monolayers, these clusters can be detected through AFM. [68,69] (Figure 2). Through the use of fluorescent analogs of PI(4,5)P2 , calcium-induced clusters were shown to occur in model membranes at physiologically relevant concentrations of both calcium and PI(4,5)P2 [70]. Other phosphoinositides have also shown some propensity to form cation-induced clusters. PI(3,5)P2 has been found to form nanodomains by itself in the presence of physiological concentrations of calcium cations, however, in the presence of magnesium clustering was negligible [71]. On top of that, the clusters formed by PI(3,5)P2 were much smaller and likely less stable than those formed by PI(4,5)P2 [71]. On the other hand, when the monophosphorylated PI(4)P was tested in the same type of experiments, no calcium-induced clusters were observed [71].
Figure 2. Snapshots of experiments on mixed lipid monolayers, containing different mol % of 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC) and PI(4,5)P2, while exposed to calcium. Reprinted from Biophysical Journal, 101, Ellenbroek, W.G.; Wang, Y.H.; Christian, D.A.; Discher, D.E.; Janmey, P.A.; Liu, A.J. Divalent cation-dependent formation of electrostatic PIP2 clusters in lipid monolayers. 2178–2184, Copyright (2011), with permission from Elsevier [69].
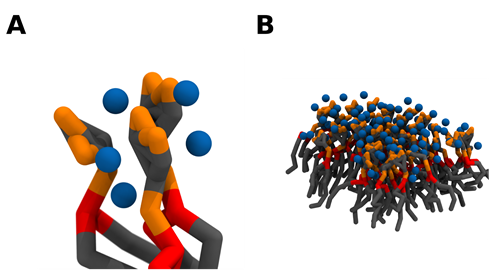
From a molecular point of view, a single divalent cation can likely crosslink up to 2 PI(4,5)P2 lipids by simultaneously binding each lipid phosphodiester and/or headgroup phosphomonoester groups via strong electrostatic interactions [72]. A single PI(4,5)P2 lipid, however, can simultaneously bind up to 3 divalent cations, and thus be complexed with 3 other PI(4,5)P2 lipids (Figure 3). This net of PI(4,5)P2 – cation interactions can thus induce the formation of a grid of tightly crosslinked lipids. Whilst the main driving force behind the clustering appears to be cation crosslinking, the formation of a complex network of intermolecular hydrogen bonds, between the headgroup hydroxyl and phosphomonoester groups, very likely plays a role in thermodynamically favoring clustering. Due to the electrostatic nature of the cation interactions, the propensity to crosslink PI(4,5)P2 lipids appears to be highly correlated with the affinity towards the divalent cation. Thus, Ca2+ shows a much greater clustering propensity than magnesium. In fact, Ca2+ induced clusters have been shown to be significantly larger than those induced by magnesium at the same experimental conditions [68]. However, although magnesium has a much weaker affinity for PI(4,5)P2 when compared to calcium, its steady-state levels are several orders of magnitude higher than those of calcium, and at these mM concentrations, it is also able to induce comparable PI(4,5)P2 clustering [71].
As the formation of these clusters is driven mainly by the crosslinking of the phosphate groups, the nanodomains formed are composed of almost only PI(4,5)P2. Studies have shown that other phosphorylated PI species can co-cluster with PI(4,5)P2, albeit to a lesser degree, but that the parent lipid phosphatidylinositol cannot [73]. Incorporation into clusters also seems to be independent from acyl-chain composition [73], however, it is very likely that different acyl-chain compositions induce the formation of nanodomains with different biophysical properties.
Figure 3. Crosslinking of PI(4,5)P2 lipids induces the formation of PI(4,5)P2 nanodomains. As a single divalent cation can bind up to 2 PI(4,5)P2 lipids and each lipid can potentially bind 3 divalent cations, a network of electrostatic interactions can crosslink PI(4,5)P2 lipids together (A). As the number of clustered lipids increases, PI(4,5)P2 nanodomains are formed (B). Coarse grain beads representing the inositol headgroup and acyl-chains are colored in grey, the glycerol component in red, the phosphate groups in orange, and calcium in blue. Snapshots obtained from a simulation of a bilayer consisting of 95:5 mol ratio POPC: PI(4,5)P2 in the presence of calcium in a 5:1 calcium to PI(4,5)P2 ratio, using the martini 2.2 coarse-grained forcefield run in GROMACS2019. Images were modeled using VMD.
These cation-induced PI(4,5)P2 nanodomains are much more than simply the sum of their elements. While calcium is known to directly regulate the interaction of different protein domains to PI(4,5)P2 [74], the structure and dynamics of the phospholipid within the divalent cation-induced cluster present markedly distinct biophysical characteristics than the monodisperse lipid. As mentioned previously, binding of divalent cations, and in particular calcium, can alter PI(4,5)P2 headgroup exposure leading to a decrease in solvent-accessible area [31]. Additionally, as divalent cations accumulate, significant screening of the headgroup charges occurs, essentially shielding the large negatively charged headgroup from potential binding partners [75]. As PI(4,5)P2 lipids are forced to accumulate in an enclosed area, further reorganization of the headgroups occurs, promoted by the molecular interactions of the divalent cations with the 3 phosphate groups [76], effectively altering the headgroup conformation. This local accumulation likely influences PI(4,5)P2 acyl-chain dynamics and, therefore, local membrane order. Studies have shown that PI(4,5)P2 nanodomains have significantly reduced lateral dynamics [70] and that PI(4,5)P2, which as a single lipid has a strong preference for disordered domains, displays significantly less affinity for disordered domains upon clustering [71]. All of these altered biophysical properties can, and most likely will, influence downstream PI(4,5)P2 signaling by modulating its interactions with protein and lipid partners.
Altogether, these findings show that divalent cation-mediated clustering can lead to the formation of specific sites in the membrane highly enriched in PI(4,5)P2 while depleting the rest of the membrane [70]. PI(4,5)P2 is likely constitutively clustered in the membrane, crosslinked by Mg2+ ions alongside other minor phosphoinositide lipids. In the vicinity of active calcium channels, where calcium concentrations increase significantly upon opening of a channel, both ions will simultaneously contribute to the formation of the nanodomains, to form larger and more stable PI(4,5)P2 nanodomains. These cation-induced nanodomains can influence not only PI(4,5)P2 lateral organization and biophysical properties but also the way proteins interact with PI(4,5)P2, by modulating their localization in the plasma membrane, their target recognition and binding affinity to PI(4,5)P2, and even further downstream interactions with other proteins. Therefore, beyond the impact of calcium on PI(4,5)P2 levels in the membrane through activation of phospholipase activity, the direct interaction of divalent cations with PI(4,5)P2 is expected to play a crucial role in the regulation of the biological activity of this phospholipid.
2.3. Effect of Cholesterol on PI(4,5)P2 Properties and Distribution
Cholesterol is a crucial membrane component, implicated in a myriad of membrane processes. However, its most noted role is in the regulation of plasma membrane biophysical properties as a “fluidity buffer”. Whilst its effects can vary with different cholesterol contents, cholesterol, in general, decreases membrane fluidity by increasing lipid packing even leading to the cholesterol-dependent formation of coexisting liquid phases [77]. Like all the other phospholipids in the plasma membrane, PI(4,5)P2 is also subject to these cholesterol-dependent effects.
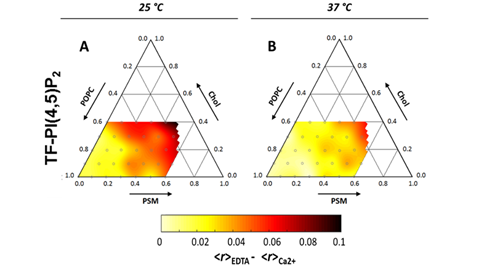
Unsurprisingly, given its large negatively charged headgroup and highly unsaturated acyl chain, PI(4,5)P2 was shown to preferentially partition into the less ordered cholesterol-poor phases of biphasic monolayers containing PI(4,5)P2:SOPC:Chol [78]. However, after the addition of calcium, the subsequent cation-induced PI(4,5)P2 nanodomains were shown to have increased the miscibility of the coexisting domains in the cholesterol-containing monolayers [78]. Related results have been observed, in a study with fluorescent derivatives of PI(4,5)P2 incorporated in ternary mixtures of POPC:SM:Chol. In this study, monodisperse PI(4,5)P2 presented low miscibility in more ordered lipid phases, yet, after cation-induced clustering, the preference for disordered domains decreased by more than two-fold [78]. Importantly, the lipid composition of this ternary mixture was shown to have a marked influence both on the extent of PI(4,5)P2 calcium-induced clustering and on the size of clusters formed (Figure 4) [78]. Since the dimensions of PI(4,5)P2 clusters were heavily dependent on temperature, it was concluded that the major factor regulating PI(4,5)P2 clustering was membrane order and not the presence of a specific molecular partner in the membrane. This suggests that the insertion of PI(4,5)P2 in more ordered domains is stabilized by the formation of cation-induced nanodomains. In a cellular context, the effect of cholesterol on PI(4,5)P2 appears to be heavily dependent on cell type. In fibroblasts [79] and cultured pancreatic β-cells [80], cholesterol depletion leads to decreased levels of free PI(4,5)P2, whilst in HEK293[81] cholesterol enrichment was shown to promote PI(4,5)P2 depletion.
Figure 4. Ternary diagram for the POPC:PSM:Chol lipid mixture at 25 °C (A) and 37 °C (B). Color-code depicts decrease in measured fluorescence anisotropies of a PI(4,5)P2 fluorescent analog (TF-PI(4,5)P2) upon inclusion of 100 µM Ca2+. Since the decrease reflects homo-FRET between analogs incorporated within the same clusters, darker areas correspond to more efficient PI(4,5)P2 clustering. Adapted with permission from Sarmento, M.J.; Coutinho, A.; Fedorov, A.; Prieto, M.; Fernandes, F. Membrane order is a key regulator of divalent cation-induced clustering of PI(3,5)P2 and PI(4,5)P2. Langmuir 2017, 33, 12463–12477 [78]. Copyright (2017) American Chemical Society.
2.4. Effect of the Cytoskeleton and Curvature on PI(4,5)P2 Lateral Organization
PI(4,5)P2 has been shown to be a major player in cytoskeleton dynamics, by interacting and regulating the activity of numerous enzymes and cytoskeletal proteins [89,90]. However, the cytoskeleton can also regulate PI(4,5)P2, and in particular its lateral organization, via corralling by the cortical actin network. Cortical actin networks have been shown to be able to induce spatio-temporal confinement of phospholipids in the plasma membrane of living cells [91]. PI(4,5)P2 should be no exception to this effect and, in fact, due to its close proximity with a variety of actin-binding proteins [90], one can suspect it could be even more susceptible to these effects. Studies have shown that the cytoskeleton is responsible for some of the low mobility of PI(4,5)P2 in atrial myocytes [92].
Curvature can also greatly influence PI(4,5)P2 lateral organization. PI(4,5)P2 has been found to undergo a transient increase at the phagocytic cup during the initiation of phagocytosis [93,94]. More recently, it was found that the curvature induced by the engagement of non-biological solid particles with the plasma membrane was enough to increase PI(4,5)P2 concentrations at the site of contact. Additionally, as we previously discussed, PI(4,5)P2 has been associated with several stages of endocytosis and exocytosis, where curvature effects are paramount [40]. As a monodisperse lipid, PI(4,5)P2 has an inverted cone-shaped structure [95] due to its very large inositol headgroup. As such, it is associated with positive membrane curvature. After interacting with divalent cations, however, PI(4,5)P2 presents a cone-shaped structure [95], likely due to the decrease in headgroup area as well as the aggregation of the headgroups after complexation with the cations. In this case, it would be associated with negative membrane curvature. Whether local curvature at the plasma membrane plays a major role in dictating PI(4,5)P2 lateral organization or PI(4,5)P2 lateral organization contributes to local curvature is not entirely clear. In a cellular context, it is likely dependent on the process in question and the overall result of both effects.
This entry is adapted from the peer-reviewed paper 10.3390/molecules25173885