Antibiotics belong to different classes of chemicals—including those of biological, synthetic, or semi-synthetic origin—and have selective modes of action. Based on their mechanisms of action, antimicrobial compounds are classified into two groups: bacteriostatic and bactericidal. Resistance is a natural adaptive tool that offers selection pressure to bacteria, and hence cannot be stopped entirely but rather be slowed down. Antibiotic resistance mutations mostly diminish bacterial reproductive fitness in an environment without antibiotics; however, a fraction of resistant populations ‘accidentally’ emerge as the fittest and thrive in a specific environmental condition, thus favouring the origin of a successful resistant clone.
- antibiotic resistance
- bacteriostatic
- Evolutionary Microbial Genetics
1. Introduction
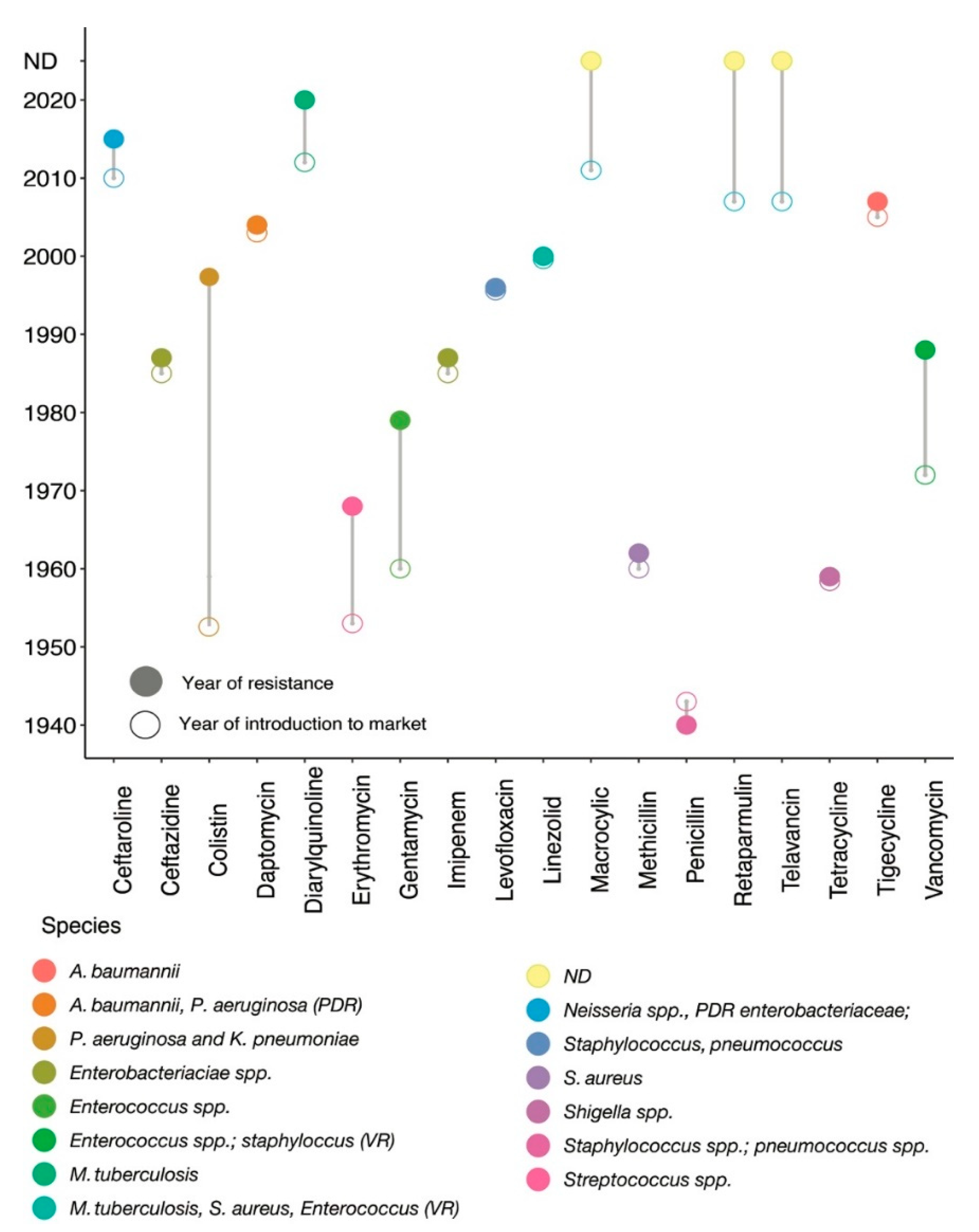
2. Antibiotics and Their Impact on Bacterial Cellular Perturbation
|
Bacteriostatic Candidates |
Mode of Action |
Mechanism of Resistance |
|---|---|---|
|
Tetracycline |
Reversibly inhibits 30S ribosomal subunit of bacteria [14]. |
Efflux system and protecting ribosomes [15]. |
|
Macrolides |
Reversibly inhibits 50S ribosomal subunit of bacteria [16]. |
Methylation of the 23S rRNA, efflux system [17]. |
|
Sulphonamides |
Inhibits folate synthesizing enzyme dihydropteroate synthase (DHPS) [18]. |
By horizontal transfer of dihydropteroate synthase gene [19]. |
|
Streptogramins |
Reversibly inhibits 50S ribosomal subunit of bacteria [20]. |
Acetyltransferases vatD gene expression mediates streptogramin A, wheras vatE and ermB or vgbA gene cluster confers streptogramin B antibiotics [21]. |
|
Oxazolidinones |
Reversibly inhibits 50S ribosomal subunit of bacteria [22]. |
High diversity and coselection of optrA [23]. |
|
Lincosamides |
Reversibly inhibits 50S ribosomal subunit of bacteria [24]. |
Target site modification, efflux system and drug inactivation [25]. |
|
Trimethoprim |
Occupying the active site of bacterial dihydrofolate reductase (DHFR), thus blocking the activity of the enzyme [26]. |
Increase expression of DHFR or decrease the affinity of DHFR to the drug [27]. |
|
Bactericide Candidates |
Mode of Action |
Mechanism of Resistance |
|---|---|---|
|
Penicillins |
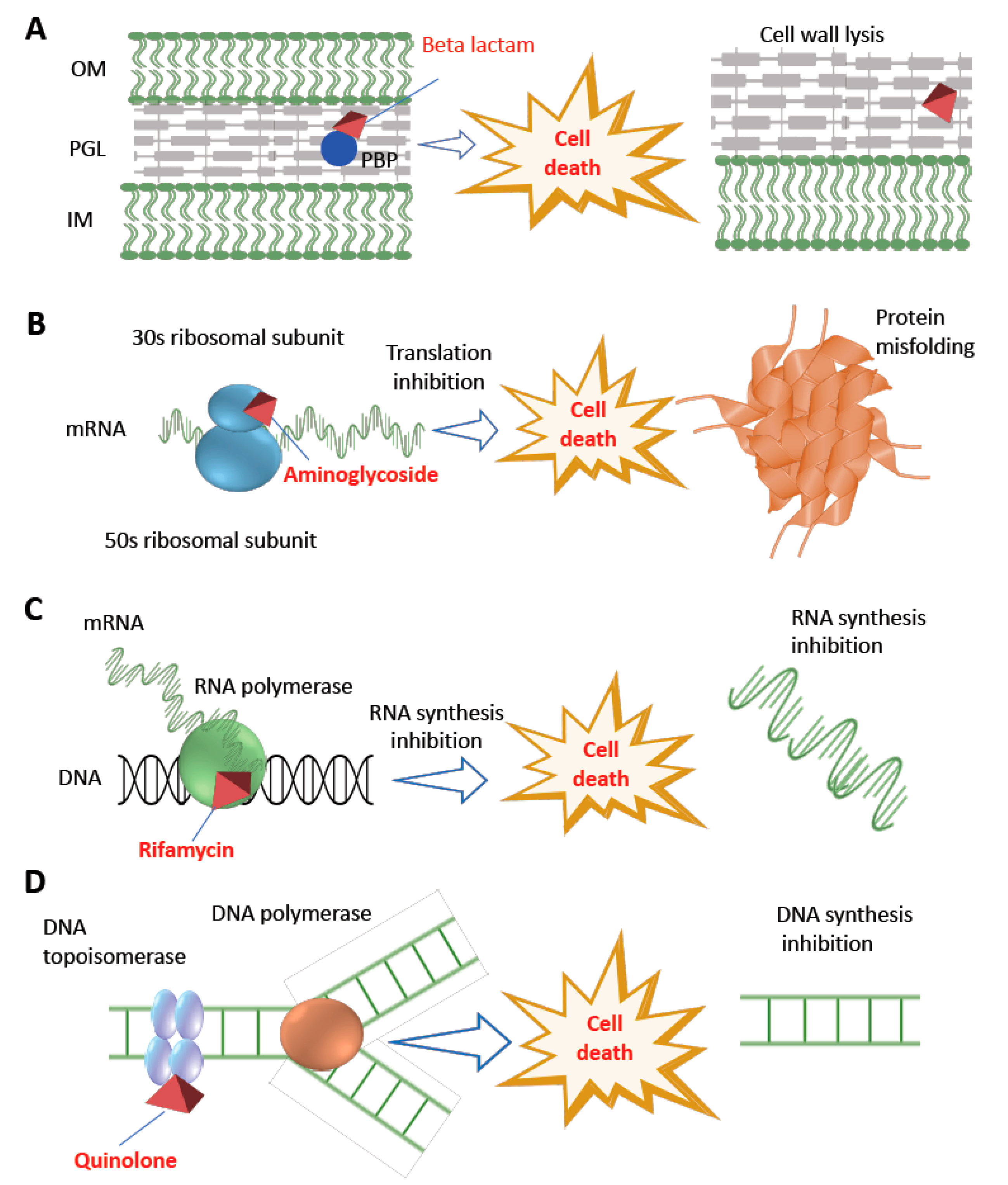
Competitively inhibits the transpeptidase enzyme resulting cross-linking blockage in cell wall [28]. |
Beta-lactamase encoded by blaZ, altered PBP2a encoded by mecA [7][29], extended-spectrum-beta-lactamases (ESBLs), AmpC beta-lactamase (i.e., blaAmpC) [30][31][32]. |
|
Cephalosporins |
Competitively inhibits the transpeptidase enzyme resulting in cross-linking or blockage in cell wall [33]. |
AmpC beta-lactamase (i.e., blaAmpC), ESBLs (i.e., blaCTX-M) [30][31]. |
|
Carbapenems |
Binding with penicillin-binding proteins (PBPs) and inactivation of these proteins leads to cell wall synthesis interruption [34]. |
Carbapenemases (i.e., class A serine-carbapenemase including KPCs; class B metallo-carbapenemase including New-Delhi-metallo-beta-lactamases or NDM, Verona-integron-encoded beta-lactamases or VIM, Imepenemase IMP-carbapenemase (also a metallo-beta-lactamase); class D serine carbapenemase such oxacillinase (OXA) [35][36], mutation-derived target enzyme modification [37]; preventing the drug entry by modifying outer membrane permeability [38]; pumping carbapenems out by efflux pump systems [39]. |
|
Aminoglycoside |
Binding with 30 s ribosomal subunit resulting genetic code misreading followed by interruption of bacterial translation [40]. |
Mostly through aminoglycosides modifying enzymes encoded by aac (aminoacetyl-tranferase) and aph (aminophospho-transferase), efflux system, or mutation in rpsL and 16S rRNA [32][41]. |
|
Fluoroquinolones |
Interrupting bacterial DNA replication by inhibiting topoisomerases [42]. |
Target enzyme mutation (DNA gyrase encoded by gyrA and gyrB, and topoisomerase IV encoded by parC and parE genes), efflux system and changing drug entry [43]. |
|
Rifamycin |
Interrupting transcription by inhibiting bacterial RNA polymerase [44]. |
Mutation of the target (beta subunit of RNA polymerase encoded by rpoB) [45]. |
|
Polymyxins |
Binding to lipid A of LPS and interfere with outer membrane permeability [46]. |
The pmrHFIJKLM (also known as arn operon) and pmrCAB operon—both invove in the biosynthesis of LAra4N and modify the lipid A, thus disrupt lipid A charges [10]; mutations in genes encoding the two-component regulatory systems such as pmrAB [47], phoPQ and plasmid-borne mcr genes confer resistance to colistin—the last line of drug [48][49]. |
|
Daptomycin |
Binding to anionic phospholipids in the cytoplasmic membrane [50]. |
Mutations in gene mprF which encodes the multiple peptide resistance factor [51]. |
|
Vancomycin |
Binding to the dipeptide terminus d-Ala-d-Ala of peptidoglycan pentapeptide precursors preventing peptidoglycan crosslinking leads to the inhibition of bacterial cell wall synthesis [52]. |
Replacing d-Ala-d-Ala with d-Ala-d-lac or d-Ala-d-Ser alternatives to which vancomycin has low affinity [53]. |
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11010040
References
- Lawrie, R. First clinical use of penicillin. Br. Med. J. 1985, 290, 397.
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Levy, S.B. Antibiotic Resistance: Consequences of Inaction. Clin. Infect. Dis. 2001, 33, S124–S129.
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283.
- Kwon, J.H.; Powderly, W.G. The post-antibiotic era is here. Science 2021, 373, 471.
- Abraham, E.P.; Chain, E. An Enzyme from Bacteria able to Destroy Penicillin. Nature 1940, 146, 837.
- Lobanovska, M.; Pilla, G. Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Med. 2017, 90, 135–145.
- Rammelkamp, C.H.; Maxon, T. Resistance of Staphylococcus aureus to the Action of Penicillin. Proc. Soc. Exp. Biol. Med. 1942, 51, 386–389.
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti. Infect. Ther. 2012, 10, 917–934.
- Falagas, M.E.; Rafailidis, P.I.; Matthaiou, D.K. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist. Updates 2010, 13, 132–138.
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132.
- Davis, B.D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 1987, 51, 341–350.
- Walsh, C. Antibiotics; American Society of Microbiology: Washington, DC, USA, 2003.
- Smilack, J.D. The Tetracyclines. Mayo Clin. Proc. 1999, 74, 727–729.
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260.
- Smieja, M. Current indications for the use of clindamycin: A critical review. Can. J. Infect. Dis. 1998, 9, 22–28.
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, a025395.
- Brock Madigan, M.T.; Martinko, J.M.; Stahl, D.A.; Clark, D.P. Brock Biology of Microorganisms, 13th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009; ISBN 1402472633.
- Sköld, O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Update 2000, 3, 155–160.
- Vannuffel, P.; Cocito, C. Mechanism of action of streptogramins and macrolides. Drugs 1996, 51, 20–30.
- Werner, G.; Klare, I.; Witte, W. Molecular analysis of streptogramin resistance in enterococci. Int. J. Med. Microbiol. 2002, 292, 81–94.
- Pandit, N.; Singla, R.K.; Shrivastava, B. Current Updates on Oxazolidinone and Its Significance. Int. J. Med. Chem. 2012, 2012, 159285.
- Chen, H.; Wang, X.; Yin, Y.; Li, S.; Zhang, Y.; Wang, Q.; Wang, H. Molecular characteristics of oxazolidinone resistance in enterococci from a multicenter study in China. BMC Microbiol. 2019, 19, 162.
- Schlünzen, F.; Zarivach, R.; Harms, J.; Bashan, A.; Tocilj, A.; Albrecht, R.; Yonath, A.; Franceschi, F. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature 2001, 413, 814–821.
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002, 34, 482–492.
- Manna, M.S.; Tamer, Y.T.; Gaszek, I.; Poulides, N.; Ahmed, A.; Wang, X.; Toprak, F.C.R.; Woodard, D.R.; Koh, A.Y.; Williams, N.S.; et al. A trimethoprim derivative impedes antibiotic resistance evolution. Nat. Commun. 2021, 12, 2949.
- Tamer, Y.T.; Gaszek, I.K.; Abdizadeh, H.; Batur, T.A.; Reynolds, K.A.; Atilgan, A.R.; Atilgan, C.; Toprak, E. High-Order Epistasis in Catalytic Power of Dihydrofolate Reductase Gives Rise to a Rugged Fitness Landscape in the Presence of Trimethoprim Selection. Mol. Biol. Evol. 2019, 36, 1533–1550.
- Yocum, R.R.; Rasmussen, J.R.; Strominger, J.L. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J. Biol. Chem. 1980, 255, 3977–3986.
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273.
- Jacoby, G.A. AmpC β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182.
- Canton, R.; Gonzalez-Alba, J.M.; Galán, J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012, 3, 110.
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415.
- Yotsuji, A.; Mitsuyama, J.; Hori, R.; Yasuda, T.; Saikawa, I.; Inoue, M.; Mitsuhashi, S. Mechanism of action of cephalosporins and resistance caused by decreased affinity for penicillin-binding proteins in Bacteroides fragilis. Antimicrob. Agents Chemother. 1988, 32, 1848–1853.
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960.
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272.
- Marie, Q.A.; Karen, B. Carbapenemases: The Versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458.
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2015, 3, 15–21.
- Bonomo, R.A.; Szabo, D. Mechanisms of Multidrug Resistance in Acinetobacter Species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43, S49–S56.
- Walsh, T.R. Emerging carbapenemases: A global perspective. Int. J. Antimicrob. Agents 2010, 36, S8–S14.
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029.
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. Medchemcomm 2016, 7, 11–27.
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574.
- Jacoby, G.A. Mechanisms of Resistance to Quinolones. Clin. Infect. Dis. 2005, 41, S120–S126.
- Wehrli, W. Rifampin: Mechanisms of Action and Resistance. Rev. Infect. Dis. 1983, 5, S407–S411.
- Tupin, A.; Gualtieri, M.; Roquet-Banères, F.; Morichaud, Z.; Brodolin, K.; Leonetti, J.-P. Resistance to rifampicin: At the crossroads between ecological, genomic and medical concerns. Int. J. Antimicrob. Agents 2010, 35, 519–523.
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916.
- Bingbing, S.; Haiyan, L.; Yu, J.; Lei, S.; Sheng, Y.; Daijie, C.; Mariana, C. New Mutations Involved in Colistin Resistance in Acinetobacter baumannii. mSphere 2021, 5, e00895-19.
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin update on its mechanism of action and resistance, present and future challenges. Microorganisms 2020, 8, 1716.
- Laurent, P.; Aurélie, J.; Patrice, N. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596.
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The Target of Daptomycin Is Absent from Escherichia coli and Other Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639.
- Mishra, N.N.; Yang, S.-J.; Chen, L.; Muller, C.; Saleh-Mghir, A.; Kuhn, S.; Peschel, A.; Yeaman, M.R.; Nast, C.C.; Kreiswirth, B.N.; et al. Emergence of Daptomycin Resistance in Daptomycin-Naïve Rabbits with Methicillin-Resistant Staphylococcus aureus Prosthetic Joint Infection Is Associated with Resistance to Host Defense Cationic Peptides and mprF Polymorphisms. PLoS ONE 2013, 8, e71151.
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.; Mark, A.E.; Cooper, M.A. Developments in Glycopeptide Antibiotics. ACS Infect. Dis. 2018, 4, 715–735.
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669.
- Drlica, K.; Malik, M. Fluoroquinolones: Action and Resistance. Curr. Top. Med. Chem. 2003, 3, 249–282.
- Tomasz, A. The mechanism of the irreversible antimicrobial effects of penicillins: How the beta-lactam antibiotics kill and lyse bacteria. Annu. Rev. Microbiol. 1979, 33, 113–137.
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810.
- Kohanski, M.A.; Dwyer, D.J.; Wierzbowski, J.; Cottarel, G.; Collins, J.J. Mistranslation of Membrane Proteins and Two-Component System Activation Trigger Antibiotic-Mediated Cell Death. Cell 2008, 135, 679–690.
- Floss, H.G.; Yu, T.W. Rifamycin—Mode of action, resistance, and biosynthesis. Chem. Rev. 2005, 105, 621–632.
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912.
- McClure, W.R.; Cech, C.L. On the mechanism of rifampicin inhibition of RNA synthesis. J. Biol. Chem. 1978, 253, 8949–8956.
- Vakulenko, S.B.; Mobashery, S. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 2003, 16, 430–450.
- Dunkle, J.A.; Xiong, L.; Mankin, A.S.; Cate, J.H.D. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. USA 2010, 107, 17152–17157.
- Nissen, P.; Hansen, J.; Ban, N.; Moore, P.B.; Steitz, T.A. The Structural Basis of Ribosome Activity in Peptide Bond Synthesis. Science 2000, 289, 920–930.
- Menninger, J.R.; Otto, D.P. Erythromycin, carbomycin, and spiramycin inhibit protein synthesis by stimulating the dissocation of peptidyl-tRNA from ribosomes. Antimicrob. Agents Chemother. 1982, 21, 811–818.
- Patel, U.; Yan, Y.P.; Hobbs, F.W.; Kaczmarczyk, J.; Slee, A.M.; Pompliano, D.L.; Kurilla, M.G.; Bobkova, E.V. Oxazolidinones Mechanism of Action: Inhibition of the First Peptide Bond Formation. J. Biol. Chem. 2001, 276, 37199–37205.
- Karimi, R.; Ehrenberg, M. Dissociation Rate of Cognate Peptidyl-tRNA from the A-Site of Hyper-Accurate and Error-Prone Ribosomes. Eur. J. Biochem. 1994, 226, 355–360.
- Falagas, M.E.; Kasiakou, S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341.
- Gurjar, M. Colistin for lung infection: An update. J. Intensive Care 2015, 3, 3–12.
- Ernst, C.M.; Peschel, A. MprF-mediated daptomycin resistance. Int. J. Med. Microbiol. 2019, 309, 359–363.
