Steroids are a group of compounds to which a number of crucial metabolism-controlling hormones belong. The group of steroid hormones that are present and active in animals and humans (mammalian steroid hormones) is large and includes, among others, corticosteroids, which control, for example, water and mineral management and sex hormones—i.e., androgens, estrogens, progesterone, which are responsible for development and reproduction. Ecdysteroids are mainly known as being the steroid hormones of arthropods that regulate ecdysis and development. In plants, the steroid regulators include the brassinosteroids, which are hormones that have a multidirectional activity and are engaged in plant growth, development, and its response to environmental stresses.
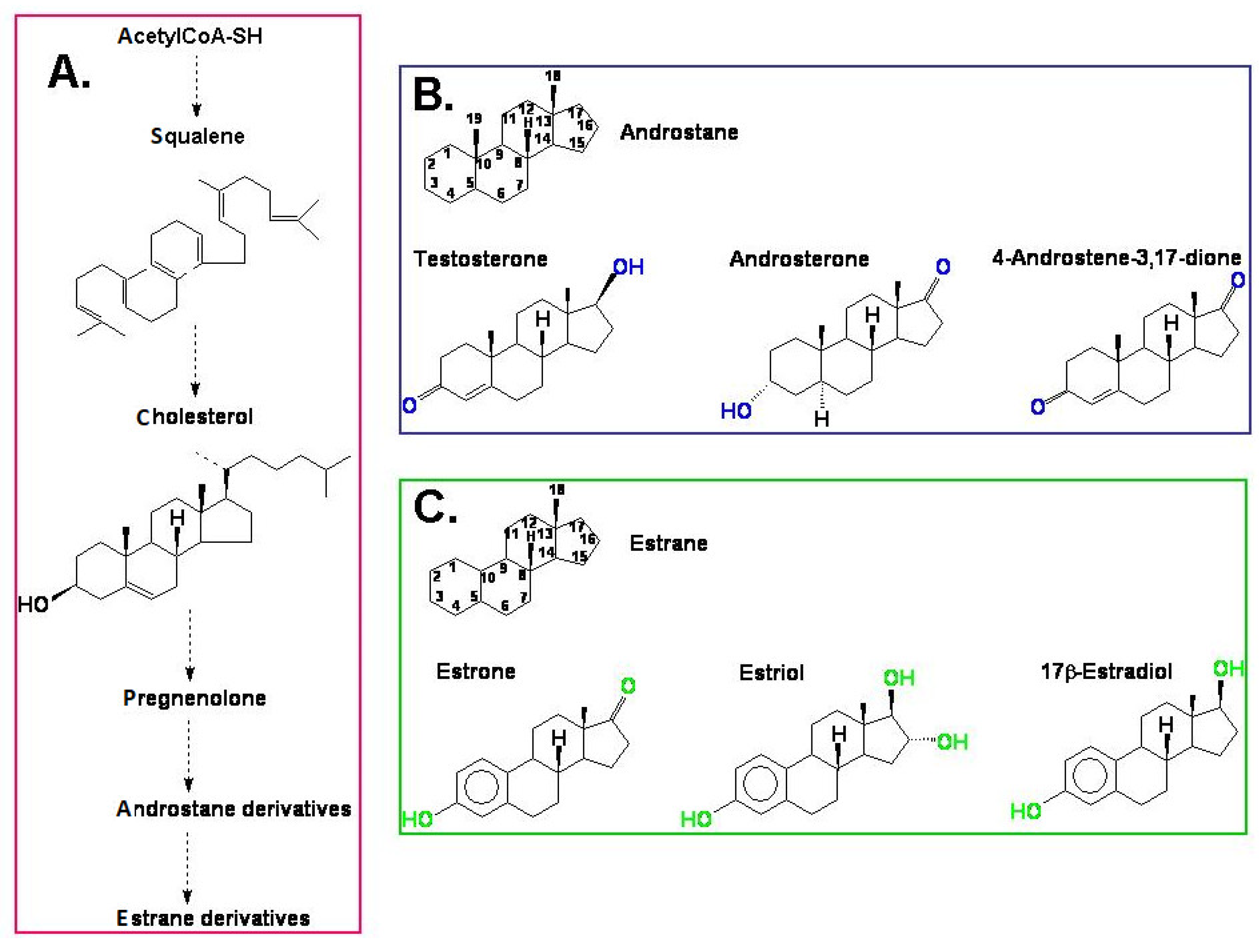
- testosterone
- androsterone
- androstenedione
- estradiol
- estrone
- plants
1. Introduction—Steroid Hormones in Living Organisms
2. The Presence of Estrogens and Androgens in Plants: The Effect of Plant Growth Conditions and Plant Developmental Stage on the Steroid Content
3. Physiological Activity of Estrogens and Androgens in Plants
3.1. Plant Growth and Reproduction
3.1.1. Plant Growth
3.1.2. Plant Reproduction
3.2. Plant Stress Response
4. Transport and Conversion of Estrogens and Androgens in Plants
5. Receptors (Specific Binding Sites) of Estrogens and Androgens in Plants
This entry is adapted from the peer-reviewed paper 10.3390/plants10122783
References
- McEwan, I.J.; Brinkmann, A.O. Androgen physiology: Receptor and metabolic disorders. In Endotext; Feingold, K., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021.
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207.
- Rochira, V.; Madeo, B.; Diazzi, C.; Zirilli, L.; Daniele, S.; Carani, C. Estrogens and male reproduction. In Endotext; Feingold, K., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021.
- Uryu, O.; Ameku, T.; Niwa, R. Recent progress in understanding the role of ecdysteroids in adult insects: Germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool. Lett. 2015, 1, 32.
- Sadura, I.; Janeczko, A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol. Plant. 2018, 62, 601–616.
- Nolan, T.M.; Vukasinovic, N.; Liu, D.R.; Russinova, E.; Yin, Y.H. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318.
- Janeczko, A.; Oklest’kova, J.; Siwek, A.; Dziurka, M.; Pociecha, E.; Kocurek, M.; Novak, O. Endogenous progesterone and its cellular binding sites in wheat exposed to drought stress. J. Steroid Biochem. Mol. 2013, 138, 384–394.
- Tarkowská, D. Plants are capable of synthesizing animal steroid hormones. Molecules 2019, 24, 2585.
- Heftmann, E. Functions of steroids in plants. Phytochemistry 1975, 14, 891–901.
- Geuns, J.M. Steroid hormones and plant growth and development. Phytochemistry 1978, 17, 1–14.
- Hewitt, S.; Hillman, J.R.; Knights, B.A. Steroidal estrogens and plant-growth and development. New Phytol. 1980, 85, 329–350.
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79.
- Speranza, A. Into the world of steroids: A biochemical “keep in touch” in plants and animals. Plant Signal. Behav. 2010, 5, 940–943.
- Islam, M.T. Mammalian hormones in plants and their roles in plant-peronosporomycete interactions. Phytochemistry 2014, 12, 89–106.
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47.
- Skarżynski, B. An oestrogenic substance from plant material. Nature 1933, 131, 766.
- Simerský, R.; Novak, O.; Morris, D.A.; Pouzar, V.; Strnad, M. Identification and quantification of several mammalian steroid hormones in plants by UPLC-MS/MS. J. Plant Growth Regul. 2009, 28, 125–136.
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Analysis of bisphenol A, nonylphenol, and natural estrogens in vegetables and fruits using gas chromatography−tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 84–89.
- Capriotti, A.L.; Cavaliere, C.; Colapicchioni, V.; Piovesana, S.; Samperi, R.; Lagana, A. Analytical strategies based on chromatography-mass spectrometry for the determination of estrogen-mimicking compounds in food. J. Chromatogr. A 2013, 1313, 62–77.
- Rinaldi, S.; Déchaud, H.; Biessy, C.; Morin-Raverot, V.; Toniolo, P.; Zeleniuch-Jacquotte, A.; Akhmedkhanov, A.; Shore, R.E.; Secreto, G.; Ciampi, A.; et al. Reliability and validity of commercially available, direct radioimmunoassays for measurement of blood androgens and estrogens in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 757–765.
- Zeitoun, M.; Alsoqeer, A.-R. Detection of sex steroid hormones in alfalfa and some rangeland native species in Saudi Arabia and their subsequent effects on camel reproduction. Glob. Vet. 2014, 13, 33–38.
- Czerpak, R.; Szamrej, I. The effect of β-estradiol and corticosteroids on chlorophylls and carotenoids content in Wolffia arrhiza (L.) Wimm. (Lemnaceae) growing in municipal Białystok tap water. Pol. J. Environ. Stud. 2003, 12, 677–684.
- Brown, G.S. The Effects of Estrogen on the Growth and Tuberization of Potato Plants (Solanum tuberosum cv. ‘Iwa’) Grown in Liquid Tissue Culture Media. Master’s Thesis, University of Canterbury, Canterbury, UK, 2006.
- Bowlin, K.M. Effects of β-estradiol on Germination and Growth in Zea mays L. Master’s Thesis, Northwest Missouri State University, Maryville, MO, USA, 2014.
- Adeel, M.; Yang, Y.S.; Wang, Y.Y.; Song, X.M.; Ahmad, M.A.; Rogers, H.J. Uptake and transformation of steroid estrogens as emerging contaminants influence plant development. Environ. Pollut. 2018, 243, 1487–1497.
- Dumlupinar, R.; Genisel, M.; Erdal, S.; Korkut, T.; Taspinar, M.S.; Taskin, M. Effects of progesterone, β-estradiol, and androsterone on the changes of inorganic element content in barley leaves. Biol. Trace Elem. Res. 2011, 143, 1740–1745.
- Erdal, S.; Dumlupinar, R. Mammalian sex hormones stimulate antioxidant system and enhance growth of chickpea plants. Acta Physiol. Plant. 2011, 33, 1011–1017.
- Erdal, S.; Dumlupinar, R. Exogenously treated mammalian sex hormones affect inorganic constituents of plants. Biol. Trace Elem. Res. 2011, 143, 500–506.
- Erdal, S.; Dumlupinar, R. Progesterone and β-estradiol stimulate seed germination in chickpea by causing important changes in biochemical parameters. Z. Nat. C 2010, 65, 239–244.
- Uysal, P.; Bezirganoglu, I. Mammalian sex hormones affect regeneration capacity and enzymes activity of triticale (x Triticosecale Wittmack) in vitro culture. J. Anim. Plant Sci. 2017, 27, 1984–1992.
- Jones, J.L.; Roddick, J.G. Steroidal estrogens and androgens in relation to reproductive development in higher plants. J. Plant Physiol. 1988, 133, 156–164.
- Lashaki, M.A.; Sedaghathoor, S.; Kalatehjari, S.; Hashemabadi, D. The physiological and growth response of Petunia hybrida, Tagetes erecta and Calendula officinalis to plant and human steroids. AIMS Agric. Food 2018, 3, 85–96.
- Żabicki, P.; Rojek, J.; Kapusta, M.; Kuta, E.; Bohdanowicz, J. Effect of estrone on somatic and female gametophyte cell division and differentiation in Arabidospis thaliana cultured in vitro. Mod. Phytomorphol. 2014, 5, 25–30.
- Rojek, J.; Pawelko, L.; Kapusta, M.; Naczk, A.; Bohdanowicz, J. Exogenous steroid hormones stimulate full development of autonomous endosperm in Arabidopsis thaliana. Acta Soc. Bot. Pol. 2015, 84, 287–301.
- Ohad, N.; Yadegari, R.; Margossian, L.; Hannon, M.; Michaeli, D.; Harada, J.J.; Goldberg, R.B.; Fischer, R.L. Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell 1999, 11, 407–415.
- Figueiredo, D.D.; Batista, R.A.; Roszakt, P.J.; Hennig, L.; Kohler, C. Auxin production in the endosperm drives seed coat development in Arabidopsis. Elife 2016, 5, e20542.
- Xiong, H.; Wang, W.; Sun, M.-X. Endosperm development is an autonomously programmed process independent of embryogenesis. Plant Cell 2021, 33, 1151–1160.
- Janeczko, A.; Filek, W. Stimulation of generative development in partly vernalized winter wheat by animal sex hormones. Acta Physiol. Plant. 2002, 24, 291–295.
- Janeczko, A.; Filek, W.; Biesaga-Kościelniak, J.; Marcińska, I.; Janeczko, Z. The influence of animal sex hormones on the induction of flowering in Arabidopsis thaliana: Comparison with the effect of 24-epibrassinolide. Plant Cell Tissue Organ Cult. 2003, 72, 147–151.
- Janeczko, A.; Oklestkova, J.; Novak, O.; Śniegowska-Świerk, K.; Snaczke, Z.; Pociecha, E. Disturbances in production of progesterone and their implications in plant studies. Steroids 2015, 96, 153–163.
- Janeczko, A. The presence and activity of progesterone in the plant kingdom. Steroids 2012, 77, 169–173.
- Janeczko, A.; Biesaga-Kościelniak, J.; Dziurka, M.; Filek, M.; Hura, K.; Jurczyk, B.; Kula, M.; Oklestkova, J.; Novak, O.; Rudolphi-Skórska, E.; et al. Biochemical and physicochemical background of mammalian androgen activity in winter wheat exposed to low temperature. J. Plant Growth Regul. 2018, 37, 199–219.
- Erdal, S. Androsterone-induced molecular and physiological changes in maize seedlings in response to chilling stress. Plant Physiol. Biochem. 2012, 57, 1–7.
- Erdal, S. Alleviation of salt stress in wheat seedlings by mammalian sex hormones. J. Sci. Food Agric. 2012, 92, 1411–1416.
- Erdal, S. Exogenous mammalian sex hormones mitigate inhibition in growth by enhancing antioxidant activity and synthesis reactions in germinating maize seeds under salt stress. J. Sci. Food Agric. 2012, 92, 839–843.
- Chaoui, A.; El Ferjani, E. Heavy metal-induced oxidative damage is reduced by beta-estradiol application in lentil seedlings. Plant Growth Regul. 2014, 74, 1–9.
- Karnjanapiboonwong, A.; Chase, D.A.; Canas, J.E.; Jackson, W.A.; Maul, J.D.; Morse, A.N.; Anderson, T.A. Uptake of 17α-ethynylestradiol and triclosan in pinto bean, Phaseolus vulgaris. Ecotoxicol. Environ. Saf. 2011, 74, 1336–1342.
- Card, M.L.; Schnoor, J.L.; Chin, Y.-P. Uptake of natural and synthetic estrogens by maize seedlings. J. Agric. Food Chem. 2012, 60, 8264–8271.
- Card, M.L.; Schnoor, J.L.; Chin, Y.-P. Transformation of natural and synthetic estrogens by maize seedlings. Environ. Sci. Technol. 2013, 47, 5101–5108.
- Blackwell, B.R.; Karnjanapiboonwong, A.; Anderson, T.A.; Smith, P.N. Uptake of 17β-trenbolone and subsequent metabolite trendione by the pinto bean plant (Phaseolus vulgaris). Ecotoxicol. Environ. Saf. 2012, 85, 110–114.
- Levin, E.R. Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 2005, 19, 1951–1959.
- Leung, Y.-K.; Mak, P.; Hassan, S.; Ho, S.-M. Estrogen receptor (ER)-β isoforms: A key to understanding ER-β signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 13162–13167.
- Tanida, T.; Matsuda, K.I.; Yamada, S.; Hashimoto, T.; Kawata, M. Estrogen-related receptor β reduces the subnuclear mobility of estrogen receptor α and suppresses estrogen-dependent cellular function. J. Biol. Chem. 2015, 290, 12332–12345.
- Karamouzis, M.V.; Papavassiliou, K.A.; Adamopoulos, C.; Papavassiliou, A.G. Targeting androgen/estrogen receptors crosstalk in cancer. Trends Cancer 2016, 2, 35–48.
- Mhaouty-Kodja, S. Role of the androgen receptor in the central nervous system. Mol. Cell. Endocrinol. 2018, 465, 103–112.
- Ponnusamy, S.; Asemota, S.; Schwartzberg, L.S.; Guestini, F.; McNamara, K.M.; Pierobon, M.; Font-Tello, A.; Qiu, X.; Xie, Y.; Rao, P.K.; et al. Androgen receptor is a non-canonical inhibitor of wild-type and mutant estrogen receptors in hormone receptor-positive breast cancers. Iscience 2019, 21, 341–358.
- Milanesi, L.; Monje, P.; Boland, R. Presence of estrogens and estrogen receptor-like proteins in Solanum glaucophyllum. Biochem. Biophys. Res. Commun. 2001, 289, 1175–1179.
- Milanesi, L.; Boland, R. Presence of estrogen receptor (ER)-like proteins and endogenous ligands for ER in solanaceae. Plant Sci. 2004, 166, 397–404.
- Janeczko, A.; Budziszewska, B.; Skoczowski, A.; Dybała, M. Specific binding sites for progesterone and 17β-estradiol in cells of Triticum aestivum L. Acta Biochim. Pol. 2008, 55, 707–711.
