Spermatogenesis is a highly coordinated process that begins with division of spermatogonia, followed by meiosis to produce haploid spermatids, and finally the differentiation of haploid spermatids into mature spermatozoa. Several stages of male germ cell development are regulated by epigenetic mechanisms that are important for correct gamete development and functions. The use of Cannabis sativa has been demonstrated to induce spermatogenesis dysfunctions. Cannabis sativa (Marijuana) exerts its effect by binding to and activating cannabinoid receptors CB1 and CB2 . In males, both the receptors CB1 and CB2, are involved in male germ cell development. Here we will discuss on the importance of cannabinoid receptors signaling in the regulation of several stages of male germ cell development and their role in mediating epigenetic modifications that may be transmitted to the next generation by sperm.
- spermatogenesis
- cannabinoids
- epigenetics
- sperm
- intergenerational effect
- cannabinoid receptor
Cannabinoid receptors are members of the superfamily of seven-transmembrane-spanning receptors and are coupled with G proteins. Both cannabinoid receptors, CB1 and CB2, are implicated in male reproductive biology [1] [2]. However, they seem to have specific expression in germ cells at different stages of differentiation and distinct roles in regulating fertility.
Cannabinoid Receptor CB1
CB1 is prominently expressed in the central nervous system (CNS) and has attracted great attention as a modulator of different brain functions. It is most abundant in the hippocampus, basal ganglia, cerebellum, and prefrontal cortex and is involved in a variety of physiological functions including appetite, fear, anxiety and pain[3][4][5] . However, it has also been detected in peripheral tissues including the reproductive system. CB1 is encoded by the gene CNR1 and consists of 472 amino acids in humans, 473 amino acids in rats and mice, with 97–99% amino acid sequence identity among these species. In addition to the canonical long form, the presence of splice isoforms both in humans and mice [6], coming from 5′-UTR introns of the gene, have been described. These three isoforms are differentially expressed in the human brain, skeletal muscle, liver, and pancreatic islet[7] and via different signaling properties, contribute to the CB1 receptor physiology.
In the testis, CB1 is expressed by somatic and germ cells of mammalian and non-mammalian vertebrates and its activity is correlated to the Leydig cell differentiation, steroidogenesis, spermiogenesis, sperm maturation, and quality. In both rat and mouse, a key role for CB1 has been demonstrated in Leydig cell development, and its expression in these cells positively correlates with differentiation events and negatively with respect to their proliferation [8][9].
In mouse germ cells, CB1 mRNAs expression is detectable in fetal gonocytes starting from E11.5 and their expression level remains low and constant during embryo development and after birth [10]. A higher level of CB1 starts to be expressed during spermatogenesis in haploid cells and became more evident in sperm, indicating a role of this receptor in the final steps of germ cell differentiation such as spermiogenesis and acquisition of functional properties. It has been demonstrated that Cb1−/− male mice show inefficient histone displacement and produce spermatozoa with uncondensed chromatin and damaged DNA[11] indicating that CB1 is involved in spermiogenesis and, in particular, plays a role in chromatin remodeling by regulating histone displacement and Tnp2 expression levels.
Mouse sperm express an even higher level of CB1, and its activation causes adverse effects on sperm function including inhibition of motility, capacitation, and acrosome reaction [12]. On the other hand, in the absence of CB1 signaling, sperm acquire motility precociously and the percentage of motile spermatozoa recovered from the caput of the epididymis is higher with respect to wild-type mice, suggesting a physiological role of this receptor in controlling sperm motility in the epididymis[13] [48]. Physiologically, a gradient of the endocannabinoid 2-AG in the epididymis prevents activation of sperm motility in caput, through activation of CB1[14] . Similarly, in humans, CB1 is expressed by sperm and its activation inhibits motility by decreasing mitochondrial activity[15], while CB1 inhibition through the use of rimonabant, a CB1 antagonist, is able to increase sperm motility and viability and to induce acrosome reaction and capacitation[16].
In human sperm, CB1 receptor is localized in the plasma membranes of the head and middle piece and has been also identified intracellularly on the mitochondria membrane (mtCB1)[17][18][19]. Although the expression of functional intracellular CB1 in mitochondria has been demonstrated in other tissues such as brain [17] and skeletal muscles[18], where it can regulate cellular respiration and other bioenergetic processes[16], the role of mtCB1 in sperm is not entirely clarified. The fact that mitochondria are the principal suppliers of sperm energy and that cannabinoids are potent inhibitors of sperm mitochondrial O2 consumption [20] suggests that mtCB1 could mediate adverse effects of cannabinoid drugs on mitochondrial functionality and thereforeexplain the negative effects on sperm motility.
In human sperm cells, CB1 has been found co-localized with the vanilloid receptor TRPV1, known as the heat-sensing receptor[21] . TRPV1 is activated by temperatures higher than 42 °C[22][23] and has been suggested to be a mediator of sperm thermotaxis in humans[24] and to play a role in the stabilization of the plasma membranes in capacitated sperm[25] . Mammalian spermatozoa, immediately after ejaculation, are unable to fertilize the oocytes and acquire this competence during the transit within the female genital tract. Sperm cells undergo a series of morpho-functional modifications, known as “capacitation”[26] that allow them to become able to recognize the oocyte and to extrude the content of acrosomal vesicle (acrosome reaction, AR), thus penetrating the zona pellucida (ZP) and reaching the oocyte membrane. It has been proposed that both the receptors CB1 and TRPV1[27][28] could participate in the modulation of spermatozoa maturation allowing sperm to acquire fertilizing ability[27][29] [82,83]. Specifically, CB1 could be implicated in the Gi protein/cAMP/PKA pathway in the early stages of post ejaculation, promoting the maintenance of membrane stability and avoiding premature acrosome reaction. TRPV1, on the contrary, could be activated in the latest stages of capacitation determining the rapid increase in intracellular calcium concentration needed for acrosome reaction. The observation that TRPV1 expression, at mRNA and protein level, is not limited to human sperm cells but has been detected also in murine germ cells from spermatocyte to spermatozoa and in Sertoli cells[30][26] suggests its potential protective role against heat stress and in conferring heat resistance to male germ cells [31].
Cannabinoid receptor CB2
CB2 is referred to as the peripheral cannabinoid receptor since it is predominantly expressed in the immune system [32] where it participates in the regulation of immune responses and in mediating the anti-inflammatory effects of C. sativa[33] . However, CB2 shows a moderate expression in other peripheral tissues, including the cardiovascular system, gastrointestinal tract, liver, adipose tissue, bone, and reproductive system. More recently a functional CB2, expressed in neurons of the hippocampus, has been identified[34]. CB2 is encoded by the gene CNR2 and consists of 360 amino acids in humans. Two isoforms of the CB2 have been identified in humans: hCB2A and hCB2B. Strikingly, these two isoforms show a tissue-specific expression: hCB2A is mainly expressed in the testis, more than 100-fold than in spleen and leukocytes, whereas the other hCB2B is expressed predominantly in spleen and at lower level in other peripheral tissues except the testis[35] . The expression of the testis-specific isoform might indicate that hCB2A could regulate functions related to spermatogenesis and fertilization. However, detailed information on the expression and role of hCB2A in human testis to date are unknown. Agirregoitia et al. reported the expression of CB2 in human sperm and suggested that, along with CB1, it could be also involved in sperm motility regulation[36]. However, various evidence indicates that CB2 is expressed at a higher level in germ cells at early stage of differentiation in mice, rats, and humans [28][37][38]. It is already expressed by gonocytes in fetal mouse testis starting from E11.5 and its expression increases during embryo development reaching a very high level in spermatogonia at birth [8]. In postnatal mouse testis, CB2 continues to be expressed by spermatogonia and its expression dramatically decreases in spermatocytes, reaching a very low level in spermatids and disappearing in mouse spermatozoa [28]. Interestingly, spermatogonia possess also the higher level of the endocannabinoid 2-AG, which decreases in spermatocytes (~2-fold) and in spermatids (~20-fold; see Figure 1). Accordingly, spermatogonia express higher and lower levels of 2-AG biosynthetic and degrading enzymes, respectively, as compared to meiotic and postmeiotic cells. Altogether these observations indicate the involvement of an autocrine/paracrine endocannabinoid signaling mediated by CB2 receptor and sustained by 2-AG, which may regulate several functions in mitotic male germ cells. In this context, it has been demonstrated that activation of CB2, through the use of the selective agonist JWH-133, promoted in vitro meiotic entry of mouse spermatogonia [28] while it did not affect mitotic germ cell proliferation (P.G., unpublished observation). Morphological and molecular evidence supported these conclusions, since CB2 activation in spermatogonia increased: (a) the number of SYCP3 positive cells, corresponding to early meiotic prophase stages, (b) the expression of early meiotic genes, and (c) the expression of the meiosis-specific histone H3K4me3 methyltransferase Prdm9. PRDM9 trimethylates specific H3K4 sites, at meiotic entry, specifying the recombination hotspots, essential for progression through prophase I[39]. Accordingly CB2 activation in spermatogonia increases the global level H3K4me3 and induced histone modifications at promoter regions of meiotic and premeiotic genes c-Kit and Stra8, compatible with their transcriptional activation. All these events occur physiologically during spermatogenesis when B-type spermatogonia enter meiosis and reach the leptotene stage of prophase I, suggesting that CB2 could play a physiological pro–meiotic role in spermatogenesis, controlling the timely coordinated progression of spermatogenesis. Notably, chronic administration of JWH-133 to immature male mice induces an acceleration of the onset of spermatogenesis, whereas the specific CB2 antagonist delays germ cell differentiation, thus demonstrating that both hyper- and hypo-stimulation of CB2 disrupted the temporal dynamics of the spermatogenic cycles [40]. These findings highlight the importance of proper CB2 signaling in the testis for the maintenance of a correct temporal progression of spermatogenesis. Disruption of the temporal dynamics of the spermatogenic cycle has important clinical implications because it frequently leads to reduced fertility or infertility due to increased germ cell apoptosis[41] . Regarding CB2, very recently, we have demonstrated that the hyperactivation of this cannabinoid receptor in male mice, besides promoting germ cell differentiation, reduced sperm number recovered by cauda epididymis[42]. This apparent discrepancy could be explained by a loss of the accelerated germ cells caused by apoptosis. Accordingly, a similar effect has been demonstrated in fetal oocyte at meiotic entry. In females, activation of CB2 signaling in fetal oocytes exerts a pro-meiotic effect in vitro and causes, in vivo, an increase in apoptotic cell death that leads to reduced ovarian reserve at birth [8].
Role of Cannabinoid Receptors in Epigenetic Modifications during Male Germ Cell Development
Recent evidence in humans and animal models reported that activation of cannabinoid receptors, through the exposure to cannabinoids, is associated with epigenetic modifications[43]. Indeed, in vitro and in vivo experiments have reported that cannabinoid treatment induces alterations in DNA methylation and histone modifications in several cell types. In human keratinocytes, it has been demonstrated that cannabinoids regulate the expression of skin differentiation genes through DNA methylation[44][45] , while Rotter et al. reported that CB1 expression is regulated by DNA methylation in peripheral blood cells in subjects with THC dependence[46] . Along the same line, another study addressed THC-induced epigenetic changes in immune cells showing histone modifications in some genes of lymph node cells in mice [47]. Regarding the CNS, it is known that the brain is particularly vulnerable to cannabinoid exposure, which can lead to adverse effects resulting in mental health disorders. In a study in which the molecular basis for this brain vulnerability was investigated, the authors identified histone modifications in three rat brain areas (hippocampus, nucleus accumbens, and amygdala), after adolescent and adult chronic THC exposure[48] . Similarly, Tomasiewicz et al. reported an increased Penk gene expression in response to rat adolescent THC exposure associated to changes in histone methylation[49] .
The effect of cannabinoids on epigenetics has been also investigated during prenatal exposure in the developing fetus, via maternal exposure during pregnancy. A study on the immune system in mice showed that in utero exposure to THC resulted in markedly defective T cell differentiation and impaired T cell function in offspring. This immunosuppressive effect has been correlated to epigenetic mechanisms such as altered microRNA, DNA methylation, and histone modification profiles[50] . In another study, maternal cannabis use has been reported to alter the developmental regulation of mesolimbic dopamine D2 receptors in offspring through histone lysine methylation [51]. A summary of studies reporting associations between post-natal (A)/prenatal (B) exposure to cannabinoids and epigenetic alterations is shown in Table 1.
Table 1. Epigenetic changes associated to cannabinoids exposure.
|
1.A. Epigenetic changes that occur within the lifespan due to direct cannabinoids exposure. |
||||
|
Drug |
Biological Target |
Epigenetic Marks |
Associated Effects |
Reference |
|
THC |
Peripheral blood cells (human) |
CB1 and CB2 promoter methylation |
Decreased CB1 expression in blood cell |
[45] |
|
THC |
Immune cells (mouse) |
Histone modifications: - H3K4me3 - H3K9me3; - H3K27me3; - H3K36me3; - H3K9ac |
Pleiotropic effect on gene expression in immune cells |
[46] |
|
THC |
- Hippocampus - Nucleus accumbens - Amygdala (rat) |
Histone modifications: - H3K9me2,3 - H3K27me3 - H3K9ac - H3K14ac |
Vulnerability to psychiatric disorders |
[52] |
|
THC |
Adult brain (rat) |
Histone modifications (H3K4me3; H3K9me3) |
Increased Penk gene mRNA levels |
[48] |
|
THC |
Mouse myeloid-derived suppressor cells |
miRNAs |
Altered miRNA involved in myeloid expansion and differentiation |
[53] |
|
THC |
Intestine (macaque) |
miRNAs |
Induction of anti-inflammatory microRNA expression |
[54] |
|
WIN55,212-2 |
Adult mouse brain (hippocampus) |
DNA methylation |
Decreased expression of Rgs7; memory impairment |
[55] |
|
1.B. Epigenetic changes that occur during fetal life due to direct in utero cannabinoids exposure. |
||||
|
Drug |
Biological Target |
Epigenetic Modification |
Associated Effects |
Reference |
|
THC |
Adult nucleus accumbens (rat) |
Histone modification (H3K4me3; H3K9me2) |
Decreased Drd2 gene expression level |
[50] |
|
THC |
Human trophoblast cell line (BeWo) |
Increased HDAC3 expression |
Gene dysregulation during placental development |
[56] |
THC—Δ9-tetrahydrocannabinol ; WIN—WIN55,212-2 synthetic cannabinoid; CB—Cannabinoid receptor; H3K—lysin of histone 3; HDAC—Histone deacetylase; Rgs7—Regulator of G-protein signaling 7 gene; Drd2—Dopamine receptor D2 gene; Penk—Proenkephalin gene.
Differently from the somatic cell types, epigenetic modifications in the germline are especially important because they can be transmitted to the progeny. Although compelling evidence is now showing that father exposure to cannabis can induce heritable changes in the sperm epigenome, very few studies have up to now addressed this point. In in vitro experiments on isolated mouse male germ cells, we reported alteration of H3K4me3 and H3K9me2 levels at the promoters of c-Kit, Stra8 and Gfra1 genes in mouse spermatogonia treated with the CB2 agonist JWH-133[39], underlining the susceptibility of these cells to epigenetic modifications. A very interesting study of Murphy et al. showed that cannabis use in humans, and THC exposure in rats, is associated with widespread changes in sperm DNA methylation[57]. From this study, they identified hypomethylation in autism candidate gene DLGAP2 in the sperm of human and rat exposed to C. sativa. Moreover, they found the same hypomethylated state in this gene in the nucleus accumbens of rats born from THC-exposed fathers[58], strongly supporting the potential for intergenerational inheritance of altered sperm DNA methylation patterns. Some other studies are beginning to shed light on cannabis/cannabinoid-induced epigenetic modifications paternally transmitted. Szutorisz et al. reported that THC exposure of male and female adolescent rats resulted in behavioral and neurobiological abnormalities in the subsequent F1 generation as a consequence of parental germline exposure to the drug [59] and, in a different report, they showed that these defects were associated to altered gene expression in the nucleus accumbens due to modified DNA methylation [60]. Levin et al. reported that paternal THC exposure in rats induced DNA methylation alterations in sperm and this correlated to impairment in attentional performance in the offspring[61] , while, another study showed that male exposure to cannabinoids during adolescence induced stress vulnerability in the offspring and this effect was associated to increased global DNA methylation in the offspring prefrontal cortex[62]. All these studies reveal that paternal exposure to cannabis and cannabinoids is associated with various behavioural and neurobiological abnormalities in the offspring through epigenetic mechanisms transmitted by sperm cells. Very recently, we investigated the effects of paternal selective activation of CB2 on offspring. We found that chronic exposure of prepubertal male mice to CB2 agonist JWH-133 induced sperm DNA hypermethylation at paternally expressed imprinted genes Plagl1 and Peg10, important for placental development and offspring growth. The hypermethylation level in these imprinted genes correlated to decreased expression of Tet genes. Interestingly, these specific alterations in sperm epigenome were inherited by the embryonic tissues and caused defects in placental and embryonic growth [41]. Overall, these studies clearly demonstrated that paternal cannabinoid receptors overactivation can induce epigenetic alterations in male gametes that are then transmitted to the next generation with an impact on offspring health as indicated in Figure 2. A summary of studies reporting associations between parental exposure to cannabinoids before conception and epigenetic alterations transmitted to the progeny is shown in Table 2. Altogether these evidence underline the susceptibility of male germ cells to epigenetic modifications following drug exposure and highlight the critical role of sperm as key vector of inheritance.
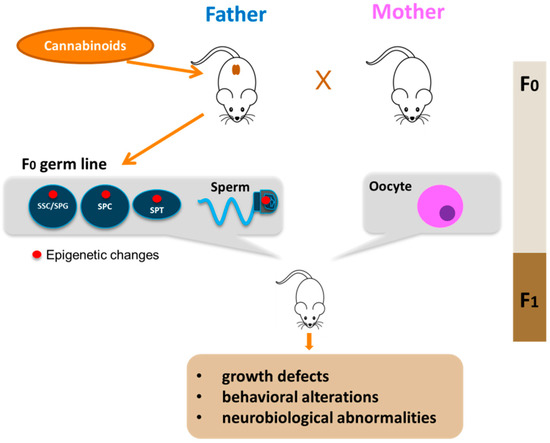
Figure 2. Paternal transmission of cannabinoid-induced epigenetic modifications. Cannabinoid exposure, particularly that during young age, leads to epigenetic alterations in the germline of the father (red circle). The epigenetic aberrations could appear in spermatogonial stem cells (SSC) or in spermatogonia (SPG) and could be maintained during germ cell differentiation in meiotic cells (SPC), haploid cells (SPT) up to sperm. Epigenetic alterations are then transmitted to F1 offspring by sperm with consequences on offspring health.
Table 2. Epigenetic changes that occur in parental germline before conception and transmitted to the F1 generation.
|
Drug |
Biological Target |
Epigenetic Modification |
Associated Effects |
Reference |
|
JWH-133 |
Spermatogonia |
Histone modification |
Accelerated entry into meiosis |
[39] |
|
THC/Cannabis |
Sperm (rat/human) |
global DNA methylation |
Altered hippo signaling and cancer pathways in sperm |
[57] |
|
Cannabis |
Sperm (rat/human) |
DNA methylation |
Hypomethylation in autism DLGAP2 gene in sperm and nucleus accumbens of offspring |
[58] |
|
THC |
Adult nucleus accumbens (rat) |
DNA methylation |
Altered methylation in genes associated with neurotransmission and synaptic plasticity genes in F1 offspring |
[60] |
|
THC |
Sperm (rat) |
DNA methylation |
Impairment in attentional performance in offspring |
[61] |
|
WIN55,212-2 |
Sperm (rat) |
DNA methylation |
Increased DNA methylation in offspring prefrontal cortex associated with stress vulnerability |
[62] |
|
JWH-133 |
Sperm (mouse) |
DNA methylation |
Hypermethylation at imprinted Peg10 and Plagl1 genes in sperm and placenta. Altered placental and embryonic growth |
[41] |
JWH—JWH-133 synthetic CB2 agonist; DLGAP2—Disks large-associated protein 2 gene;Peg10-Paternally expressed gene 10; Plagl1—PLAG1 Like Zinc Finger 1.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21010025
References
- DiPatrizio, N. V.; Piomelli, D., Intestinal lipid-derived signals that sense dietary fat. The Journal of clinical investigation 2015, 125, (3), 891-8.
- Di Marzo, V.; Stella, N.; Zimmer, A., Endocannabinoid signalling and the deteriorating brain. Nature reviews. Neuroscience 2015, 16, (1), 30-42.
- Maccarrone, M.; Bab, I.; Biro, T.; Cabral, G. A.; Dey, S. K.; Di Marzo, V.; Konje, J. C.; Kunos, G.; Mechoulam, R.; Pacher, P.; Sharkey, K. A.; Zimmer, A., Endocannabinoid signaling at the periphery: 50 years after THC. Trends in pharmacological sciences 2015, 36, (5), 277-96.
- Ruehle, S.; Wager-Miller, J.; Straiker, A.; Farnsworth, J.; Murphy, M. N.; Loch, S.; Monory, K.; Mackie, K.; Lutz, B., Discovery and characterization of two novel CB1 receptor splice variants with modified N-termini in mouse. Journal of neurochemistry 2017, 142, (4), 521-533.
- Ryberg, E.; Vu, H. K.; Larsson, N.; Groblewski, T.; Hjorth, S.; Elebring, T.; Sjogren, S.; Greasley, P. J., Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS letters 2005, 579, (1), 259-64.
- Cacciola, G.; Chioccarelli, T.; Mackie, K.; Meccariello, R.; Ledent, C.; Fasano, S.; Pierantoni, R.; Cobellis, G., Expression of type-1 cannabinoid receptor during rat postnatal testicular development: possible involvement in adult leydig cell differentiation. Biology of reproduction 2008, 79, (4), 758-65.
- Cobellis, G.; Meccariello, R.; Chianese, R.; Chioccarelli, T.; Fasano, S.; Pierantoni, R., Effects of Neuroendocrine CB1 Activity on Adult Leydig Cells. Frontiers in endocrinology 2016, 7, 47.
- De Domenico, E.; Todaro, F.; Rossi, G.; Dolci, S.; Geremia, R.; Rossi, P.; Grimaldi, P., Overactive type 2 cannabinoid receptor induces meiosis in fetal gonads and impairs ovarian reserve. Cell death & disease 2017, 8, (10), e3085.
- Chioccarelli, T.; Cacciola, G.; Altucci, L.; Lewis, S. E.; Simon, L.; Ricci, G.; Ledent, C.; Meccariello, R.; Fasano, S.; Pierantoni, R.; Cobellis, G., Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology 2010, 151, (10), 5017-29.
- Cobellis, G.; Cacciola, G.; Scarpa, D.; Meccariello, R.; Chianese, R.; Franzoni, M. F.; Mackie, K.; Pierantoni, R.; Fasano, S., Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biology of reproduction 2006, 75, (1), 82-9.
- Cobellis, G.; Ricci, G.; Cacciola, G.; Orlando, P.; Petrosino, S.; Cascio, M. G.; Bisogno, T.; De Petrocellis, L.; Chioccarelli, T.; Altucci, L.; Fasano, S.; Meccariello, R.; Pierantoni, R.; Ledent, C.; Di Marzo, V., A gradient of 2-arachidonoylglycerol regulates mouse epididymal sperm cell start-up. Biology of reproduction 2010, 82, (2), 451-8.
- Ricci, G.; Cacciola, G.; Altucci, L.; Meccariello, R.; Pierantoni, R.; Fasano, S.; Cobellis, G., Endocannabinoid control of sperm motility: the role of epididymus. General and comparative endocrinology 2007, 153, (1-3), 320-2.
- Aquila, S.; Guido, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Baffa, M. F.; Ando, S.; Bifulco, M., Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. British journal of pharmacology 2010, 159, (4), 831-41.
- Rossato, M.; Ion Popa, F.; Ferigo, M.; Clari, G.; Foresta, C., Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. The Journal of clinical endocrinology and metabolism 2005, 90, (2), 984-91.
- Benard, G.; Massa, F.; Puente, N.; Lourenco, J.; Bellocchio, L.; Soria-Gomez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; Hebert-Chatelain, E.; Mulle, C.; Ortega-Gutierrez, S.; Martin-Fontecha, M.; Klugmann, M.; Guggenhuber, S.; Lutz, B.; Gertsch, J.; Chaouloff, F.; Lopez-Rodriguez, M. L.; Grandes, P.; Rossignol, R.; Marsicano, G., Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nature neuroscience 2012, 15, (4), 558-64.
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A. C.; Delamarre, A.; Cannich, A.; Vincent, P.; Varilh, M.; Robin, L. M.; Terral, G.; Garcia-Fernandez, M. D.; Colavita, M.; Mazier, W.; Drago, F.; Puente, N.; Reguero, L.; Elezgarai, I.; Dupuy, J. W.; Cota, D.; Lopez-Rodriguez, M. L.; Barreda-Gomez, G.; Massa, F.; Grandes, P.; Benard, G.; Marsicano, G., A cannabinoid link between mitochondria and memory. Nature 2016, 539, (7630), 555-559.
- Mendizabal-Zubiaga, J.; Melser, S.; Benard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; Rodriguez De Fonseca, F.; Puente, N.; Marsicano, G.; Grandes, P., Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Frontiers in physiology 2016, 7, 476.
- Badawy, Z. S.; Chohan, K. R.; Whyte, D. A.; Penefsky, H. S.; Brown, O. M.; Souid, A. K., Cannabinoids inhibit the respiration of human sperm. Fertility and sterility 2009, 91, (6), 2471-6.
- Muller, C.; Morales, P.; Reggio, P. H., Cannabinoid Ligands Targeting TRP Channels. Frontiers in molecular neuroscience 2018, 11, 487.
- Caterina, M. J.; Schumacher, M. A.; Tominaga, M.; Rosen, T. A.; Levine, J. D.; Julius, D., The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, (6653), 816-24.
- Gavva, N. R., Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends in pharmacological sciences 2008, 29, (11), 550-7.
- De Toni, L.; Garolla, A.; Menegazzo, M.; Magagna, S.; Di Nisio, A.; Sabovic, I.; Rocca, M. S.; Scattolini, V.; Filippi, A.; Foresta, C., Heat Sensing Receptor TRPV1 Is a Mediator of Thermotaxis in Human Spermatozoa. PloS one 2016, 11, (12), e0167622.
- Maccarrone, M.; Barboni, B.; Paradisi, A.; Bernabo, N.; Gasperi, V.; Pistilli, M. G.; Fezza, F.; Lucidi, P.; Mattioli, M., Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. Journal of cell science 2005, 118, (Pt 19), 4393-404.
- Yanagimachi, R., Fertility of mammalian spermatozoa: its development and relativity. Zygote 1994, 2, (4), 371-2.
- Francavilla, F.; Battista, N.; Barbonetti, A.; Vassallo, M. R.; Rapino, C.; Antonangelo, C.; Pasquariello, N.; Catanzaro, G.; Barboni, B.; Maccarrone, M., Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology 2009, 150, (10), 4692-700.
- Bernabo, N.; Pistilli, M. G.; Mattioli, M.; Barboni, B., Role of TRPV1 channels in boar spermatozoa acquisition of fertilizing ability. Molecular and cellular endocrinology 2010, 323, (2), 224-31.
- Bernabo, N.; Palestini, P.; Chiarini, M.; Maccarrone, M.; Mattioli, M.; Barboni, B., Endocannabinoid-binding CB1 and TRPV1 receptors as modulators of sperm capacitation. Communicative & integrative biology 2012, 5, (1), 68-70.
- Grimaldi, P.; Orlando, P.; Di Siena, S.; Lolicato, F.; Petrosino, S.; Bisogno, T.; Geremia, R.; De Petrocellis, L.; Di Marzo, V., The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America 2009, 106, (27), 11131-6.
- Mizrak, S. C.; van Dissel-Emiliani, F. M., Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertility and sterility 2008, 90, (4), 1290-3.
- Mizrak, S. C.; van Dissel-Emiliani, F. M., Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertility and sterility 2008, 90, (4), 1290-3.
- Munro, S.; Thomas, K. L.; Abu-Shaar, M., Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, (6441), 61-5.
- Buckley, N. E.; McCoy, K. L.; Mezey, E.; Bonner, T.; Zimmer, A.; Felder, C. C.; Glass, M.; Zimmer, A., Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. European journal of pharmacology 2000, 396, (2-3), 141-9.
- Stempel, A. V.; Stumpf, A.; Zhang, H. Y.; Ozdogan, T.; Pannasch, U.; Theis, A. K.; Otte, D. M.; Wojtalla, A.; Racz, I.; Ponomarenko, A.; Xi, Z. X.; Zimmer, A.; Schmitz, D., Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, (4), 795-809.
- Liu, Q. R.; Pan, C. H.; Hishimoto, A.; Li, C. Y.; Xi, Z. X.; Llorente-Berzal, A.; Viveros, M. P.; Ishiguro, H.; Arinami, T.; Onaivi, E. S.; Uhl, G. R., Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes, brain, and behavior 2009, 8, (5), 519-30.
- Agirregoitia, E.; Carracedo, A.; Subiran, N.; Valdivia, A.; Agirregoitia, N.; Peralta, L.; Velasco, G.; Irazusta, J., The CB(2) cannabinoid receptor regulates human sperm cell motility. Fertility and sterility 2010, 93, (5), 1378-87.
- Migliaccio, M.; Ricci, G.; Suglia, A.; Manfrevola, F.; Mackie, K.; Fasano, S.; Pierantoni, R.; Chioccarelli, T.; Cobellis, G., Analysis of Endocannabinoid System in Rat Testis During the First Spermatogenetic Wave. Frontiers in endocrinology 2018, 9, 269.
- Nielsen, J. E.; Rolland, A. D.; Rajpert-De Meyts, E.; Janfelt, C.; Jorgensen, A.; Winge, S. B.; Kristensen, D. M.; Juul, A.; Chalmel, F.; Jegou, B.; Skakkebaek, N. E., Characterisation and localisation of the endocannabinoid system components in the adult human testis. Scientific reports 2019, 9, (1), 12866.
- Powers, N. R.; Parvanov, E. D.; Baker, C. L.; Walker, M.; Petkov, P. M.; Paigen, K., The Meiotic Recombination Activator PRDM9 Trimethylates Both H3K36 and H3K4 at Recombination Hotspots In Vivo. PLoS genetics 2016, 12, (6), e1006146.
- Di Giacomo, D.; De Domenico, E.; Sette, C.; Geremia, R.; Grimaldi, P., Type 2 cannabinoid receptor contributes to the physiological regulation of spermatogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2016, 30, (4), 1453-63.
- Busada, J. T.; Velte, E. K.; Serra, N.; Cook, K.; Niedenberger, B. A.; Willis, W. D.; Goulding, E. H.; Eddy, E. M.; Geyer, C. B., Rhox13 is required for a quantitatively normal first wave of spermatogenesis in mice. Reproduction 2016, 152, (5), 379-88.
- Innocenzi, E.; De Domenico, E.; Ciccarone, F.; Zampieri, M.; Rossi, G.; Cicconi, R.; Bernardini, R.; Mattei, M.; Grimaldi, P., Paternal activation of CB2 cannabinoid receptor impairs placental and embryonic growth via an epigenetic mechanism. Scientific reports 2019, 9, (1), 17034.
- Dobs, Y. E.; Ali, M. M., The epigenetic modulation of alcohol/ethanol and cannabis exposure/co-exposure during different stages. Open biology 2019, 9, (1), 180115.
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D'Addario, C.; Maccarrone, M., Epigenetic control of skin differentiation genes by phytocannabinoids. British journal of pharmacology 2013, 170, (3), 581-91.
- Paradisi, A.; Pasquariello, N.; Barcaroli, D.; Maccarrone, M., Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. The Journal of biological chemistry 2008, 283, (10), 6005-12.
- Rotter, A.; Bayerlein, K.; Hansbauer, M.; Weiland, J.; Sperling, W.; Kornhuber, J.; Biermann, T., CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. European addiction research 2013, 19, (1), 13-20.
- Yang, X.; Hegde, V. L.; Rao, R.; Zhang, J.; Nagarkatti, P. S.; Nagarkatti, M., Histone modifications are associated with Delta9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. The Journal of biological chemistry 2014, 289, (27), 18707-18.
- Prini, P.; Penna, F.; Sciuccati, E.; Alberio, T.; Rubino, T., Chronic Delta(8)-THC Exposure Differently Affects Histone Modifications in the Adolescent and Adult Rat Brain. International journal of molecular sciences 2017, 18, (10).
- Tomasiewicz, H. C.; Jacobs, M. M.; Wilkinson, M. B.; Wilson, S. P.; Nestler, E. J.; Hurd, Y. L., Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological psychiatry 2012, 72, (10), 803-10.
- Zumbrun, E. E.; Sido, J. M.; Nagarkatti, P. S.; Nagarkatti, M., Epigenetic Regulation of Immunological Alterations Following Prenatal Exposure to Marijuana Cannabinoids and its Long Term Consequences in Offspring. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 2015, 10, (2), 245-54.
- DiNieri, J. A.; Wang, X.; Szutorisz, H.; Spano, S. M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y. L., Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biological psychiatry 2011, 70, (8), 763-9.
- Prini, P.; Rusconi, F.; Zamberletti, E.; Gabaglio, M.; Penna, F.; Fasano, M.; Battaglioli, E.; Parolaro, D.; Rubino, T., Adolescent THC exposure in female rats leads to cognitive deficits through a mechanism involving chromatin modifications in the prefrontal cortex. Journal of psychiatry & neuroscience : JPN 2017, 42, (6), 170082.
- Hegde, V. L.; Tomar, S.; Jackson, A.; Rao, R.; Yang, X.; Singh, U. P.; Singh, N. P.; Nagarkatti, P. S.; Nagarkatti, M., Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Delta9-tetrahydrocannabinol in vivo: regulation of CCAAT/enhancer-binding protein alpha by microRNA-690. The Journal of biological chemistry 2013, 288, (52), 36810-26.
- Chandra, L. C.; Kumar, V.; Torben, W.; Vande Stouwe, C.; Winsauer, P.; Amedee, A.; Molina, P. E.; Mohan, M., Chronic administration of Delta9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute simian immunodeficiency virus infection of rhesus macaques. Journal of virology 2015, 89, (2), 1168-81.
- Tomas-Roig, J.; Benito, E.; Agis-Balboa, R. C.; Piscitelli, F.; Hoyer-Fender, S.; Di Marzo, V.; Havemann-Reinecke, U., Chronic exposure to cannabinoids during adolescence causes long-lasting behavioral deficits in adult mice. Addiction biology 2017, 22, (6), 1778-1789.
- Khare, M.; Taylor, A. H.; Konje, J. C.; Bell, S. C., Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Molecular human reproduction 2006, 12, (5), 321-33.
- Khare, M.; Taylor, A. H.; Konje, J. C.; Bell, S. C., Delta9-tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Molecular human reproduction 2006, 12, (5), 321-33.
- Murphy, S. K.; Itchon-Ramos, N.; Visco, Z.; Huang, Z.; Grenier, C.; Schrott, R.; Acharya, K.; Boudreau, M. H.; Price, T. M.; Raburn, D. J.; Corcoran, D. L.; Lucas, J. E.; Mitchell, J. T.; McClernon, F. J.; Cauley, M.; Hall, B. J.; Levin, E. D.; Kollins, S. H., Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018, 13, (12), 1208-1221.
- Schrott, R.; Acharya, K.; Itchon-Ramos, N.; Hawkey, A. B.; Pippen, E.; Mitchell, J. T.; Kollins, S. H.; Levin, E. D.; Murphy, S. K., Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 2019, 1-13.
- Szutorisz, H.; Hurd, Y. L., High times for cannabis: Epigenetic imprint and its legacy on brain and behavior. Neuroscience and biobehavioral reviews 2018, 85, 93-101.
- Watson, C. T.; Szutorisz, H.; Garg, P.; Martin, Q.; Landry, J. A.; Sharp, A. J.; Hurd, Y. L., Genome-Wide DNA Methylation Profiling Reveals Epigenetic Changes in the Rat Nucleus Accumbens Associated With Cross-Generational Effects of Adolescent THC Exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2015, 40, (13), 2993-3005.
- Levin, E. D.; Hawkey, A. B.; Hall, B. J.; Cauley, M.; Slade, S.; Yazdani, E.; Kenou, B.; White, H.; Wells, C.; Rezvani, A. H.; Murphy, S. K., Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicology and teratology 2019, 74, 106806.
- Ibn Lahmar Andaloussi, Z.; Taghzouti, K.; Abboussi, O., Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2019, 72, 48-54.
- Ibn Lahmar Andaloussi, Z.; Taghzouti, K.; Abboussi, O., Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 2019, 72, 48-54.
