Gap junctions (GJs) are intercellular junctions that allow the direct transfer of ions and
small molecules between neighboring cells, and GJs between astrocytes play an important role in the
development of various pathologies of the brain, including regulation of the pathological neuronal
synchronization underlying epileptic seizures. Recently, we found that a pathological change is
observed in astrocytes during the ictal and interictal phases of 4-aminopyridin (4-AP)-elicited epileptic
activity in vitro, which was correlated with neuronal synchronization and extracellular epileptic
electrical activity. This finding raises the question: Does this signal depend on GJs between astrocytes?
In this study we investigated the effect of the GJ blocker, carbenoxolone (CBX), on epileptic activity
in vitro and in vivo. Based on the results obtained, we came to the conclusion that the astrocytic
syncytium formed by GJ-associated astrocytes, which is responsible for the regulation of potassium,
affects the formation of epileptic activity in astrocytes in vitro and epileptic seizure onset. This effect
is probably an important, but not the only, mechanism by which CBX suppresses epileptic activity. It
is likely that the mechanisms of selective inhibition of GJs between astrocytes will show important
translational benefits in anti-epileptic therapies.
1. In Vitro Results
1.1. Depolarizing Inward Currents Synchronized with the Epileptic Activity of Neurons Is Observed in Astrocytes
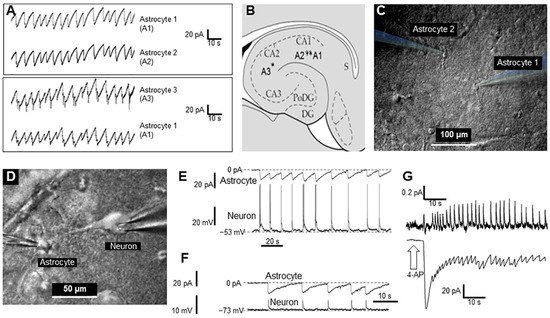
Using a dual-channel patch clamp in voltage clamp mode, it recorded membrane current in two quiescent astrocytes simultaneously. As it was described previously, after perfusion of the slice preparation for 30–60 s with 2 mM 4-AP dissolved in ACSF, the inward current oscillations started to appear in astrocytes, with a mean amplitude of 37.7 ± 2.3 pA in a single astrocyte at 0.1–0.3 Hz (
n = 27). Besides these small inward currents, short (2–3 s) but high-voltage (40–50 mV) depolarization events perturbed astrocytes periodically [
14]. Here it was presented a similar series of experiments (
n = 10) that were performed on patch pairs of astrocytes at different distances (20–600 µM) from each another. In the latter case, one astrocyte was situated in the CA1 zone and the second in the CA2 zone of the hippocampus (
Figure 1A–C). Remarkably, there was near synchronization of periodically generated inward currents in nearby astrocytes as well as in distant astrocytes, which suggests a relatively large spatial extent (≥0.6 mm) of the synchronization phenomenon. To illustrate this synchronization, the currents recorded from two pairs of astrocytes are presented in
Figure 1.
Figure 1. Synchronous activity of two astrocytes and neurons in the CA1 zone of the rat hippocampus after short-term application of 2 mM 4-AP: (A). Currents recorded from hippocampal astrocytes 1 and 2 (top panel) and 1 and 3 (bottom panel). (B). Sketch of the hippocampus with schematic positions of the astrocytes that were monitored in pairs. (C). Infrared image of two astrocytes with electrodes for patch-clamp. (D). Infrared image of a neuron and an astrocyte with electrodes attached for patch-clamp. (E,F). Current and voltage recording from an astrocyte and neuron, respectively. Panel E shows the neuron action potentials (APs), and the synchronous changes in the astrocyte’s membrane potential (Panel F) shows the excitatory postsynaptic potentials (EPSPs) of the neuron, observed during artificial hyperpolarization of the neuron, and synchronous changes in the membrane potential of an astrocyte. (G). Extracellular recording of neuron firing and current changes recorded from an astrocyte (top and bottom, respectively) elicited by 4-AP.
It also monitored neuronal activity during 4-AP application, and this was not blocked. Figure 1D,E shows a typical simultaneous recording of an astrocyte (voltage-clamp mode) and neuron (current-clamp mode), each with glass electrodes. Interestingly, activity spikes in neurons corresponded to the inward currents in astrocytes (Figure 1E).
Even when inhibiting neuronal spiking by applying a hyperpolarization current, giant excitatory synaptic currents were recorded in neurons, which were synchronized with inward current activity in astrocytes (Figure 1F). This suggests that astrocytes (and their syncytiums) respond with visible inward currents to potassium released by neurons during synchronized spikes. Moreover, it was shown that a majority of neurons (86% of all tested, n = 120) are driven by high excitatory synaptic potentials. Other neurons (14%) had no excitatory drive after application of 4-AP (not shown).
Interestingly, inward currents in astrocytes (voltage-clamp mode, Figure 1G, lower trace) corresponded to the extracellular local field potentials (LFPs) in the slice (Figure 1G, upper trace). This finding suggests that global synchronization of both neurons and astrocytes produces LFP signals in the slice and probably in whole brain.
2. CBX Inhibits GJs in Astrocytes and Blocks Epileptiformic Activity in Astrocytes in Hippocampal Slices In Vitro
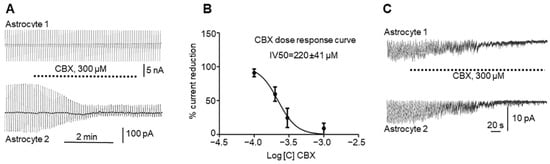
It recorded GJ currents in astrocyte pairs connected by GJs (n = 16, Figure 2A) in control animals (see Methods). It injected current in the first astrocyte of the pair (astrocyte 1, upper trace) and recorded the current that passed through the GJs to the second astrocyte in the pair (astrocyte 2, lower trace). After perfusion of the slice with 300 µM CBX (Figure 2A), the effect was a significant reduction in current in the adjacent astrocyte, which was reduced after CBX perfusion (from 284.3 ± 58.9 to 34.2 ± 7.1; paired t test, p = 0.0058) but not completely blocked. Usually, the gap current was not restored after CBX application and washout and remained low.
Figure 2. Effects of CBX on astrocyte gap junctions and on astrocyte activity elicited by 4-AP: (A). Reduction in gap junction current between astrocytes (lower curve) by application of 300 µM CBX to the bath (see text). (B). Dose–response curve of the CBX effect on inter-astrocyte gap junctions, with error bars representing SD. (C). CBX at a 300 µM concentration simultaneously reduces astrocytic “epileptiform” activity elicited by 4-AP in two astrocytes in the hippocampal slice.
It also used different concentrations of CBX and paired recordings in astrocytes to build a dose–response curve (Figure 2B), showing that 220 ± 41 µM (n = 60) CBX applied to the slice reduced the number of functional GJs by half.
Interestingly, the astrocyte “epileptiform” activity that appeared during epileptic events was affected by CBX. After perfusion of the slice with 2 mM 4-AP for 30–60 s, an astrocyte inward current was initiated. The perfusion of the slice with 300 µM CBX blocked astrocyte epileptiform activity simultaneously in all recorded astrocytes (Figure 2C), which could be restored after washout by a new perfusion with 4-AP (again, this blocking and restoring of activity can be carried out more than once).
2. In Vivo Results
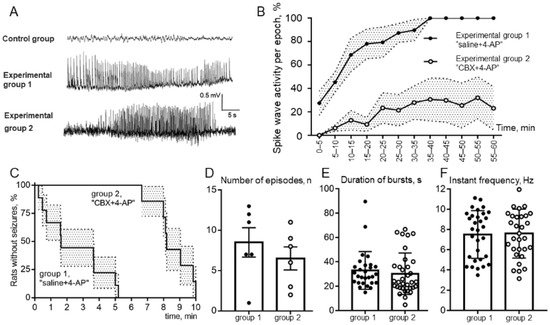
Analysis of ECoG (Figure 3A) recordings showed that none of the animals receiving only saline or CBX (the control group) showed signs of epileptiform spike-wave activity (SWA). Analysis of the behavior of animals in the experimental box after the injection of these substances into the cerebral cortex also did not reveal any pathological manifestations of epileptic convulsive motor activity. In experiments after introduction of the epileptogen 4-AP, the development of epileptiform SWA was observed, but the degree of its manifestation was different in the two experimental groups.
Figure 3. (A). Examples of a ECoG records; control group, no spike-wave activity (SWA) after only CBX or saline intracortical injection; group 1, ECoG of status epilepticus in a rat that received 4-AP after saline injection; group 2, seizure of epileptiform spike-wave activity on an ECoG of a rat that received 4-AP after CBX injection. (B). Comparison of the total duration of episodes of SWA over the epoch of analysis; (C). Percentage of animals of experimental groups 1 and 2 without seizure free during 10 min above 4-AP injection (latency to first SWA episode); (D). Comparison of the number of SWA episodes; (E). The mean values of the duration of bursts; (F). Comparison of instantaneous repetition rate in the composition of SWA episodes in rats receiving 4-AP after saline (group 1, “saline + 4-AP”) and in rats receiving 4-AP after administration of CBX (group 2, “CBX + 4-AP”). The abscissa shows the time in minutes. The mean values and errors of the mean are given, and the reliability of the differences was determined by two-way ANOVA, p < 0.0001 (B); The log-rank (Mantel–Cox) test, p < 0.0001 (C); the means ± SD, unpaired Mann–Whitney test, p > 0.05 (D–F).
The rats of group 1, which had received intracortical microinjection of an isotonic NaCl solution before the induction of epilepsy, demonstrated more severe forms of epileptiform activity. Among the rats that received 4-AP after saline injection, 100% of the animals showed SWA, and 83% (5 of 6 animals) showed the development of status epilepticus. The rats of group 2, which had previously been injected with CBX, exhibited significantly fewer manifestations of experimental epilepsy. Thus, preliminary intracortical administration of CBX significantly reduced the severity of epileptiform activity after administration of 4-AP, with only 17% (1 rat of 6) demonstrating the development of status epilepticus (Figure 3A).
Comparison of the latent periods of the onset of episodes of SWA showed (Figure 3C) a significant increase in the latency of the first episode in rats receiving 4-AP after CBX compared with rats receiving 4-AP after saline. The significance of the differences was determined using the Mann–Whitney test: the average latency was 2.46 ± 0.65 min in group 1 and 8.55 ± 0.44 min in group 2. The analysis of the percentage of rats in experimental groups 1 and 2 remaining seizure-free demonstrated that rats after intracortical injection of “saline + 4-AP” have significantly shorter latent periods of episodes of SWA than rats in the “CBX + 4-AP” group (log-rank Mantel–Cox test, hazard ratio group 1/group 2–4.62; 95% CI of ratio –1.41 to 15.13; p < 0.0001).
The mean duration and the number of epileptic episodes was calculated without considering status epilepticus in animals of experimental group 1. The durations of SWA episodes (outside of status epilepticus) in rats in both experimental groups (Figure 3E) did not differ significantly (32.9 ± 2.9 s and 30.2 ± 2.9 s, respectively). The number of epileptic episodes also did not show a significant difference (Figure 3D). It should be noted that these parameters are not a quite representative parameter for comparing groups 1 and 2, because in rats of experimental group 1, some episodes of epileptic activity were transferred to status epilepticus, representing one single prolonged episode. At the same time, the number of episodes in rats of group 2 was relatively lower due to the increased latent period of the appearance of the first episode.
To estimate the percentage of time occupied by seizures, the ECoG recording after administration of 4-AP was divided into 5 min analysis periods. It was found that rats that received the 4-AP injection followed by saline reached 100% of the total duration of CBA episodes for the epoch of analysis 35 min, on average, after administration of 4-AP, which indicates the presence of status epilepticus (Figure 3B). The rats that received intracortical administration of CBX before induction of epilepsy did not reach even 40% of the level of the total duration of SWA episodes over the epoch of analysis. Comparison by two-way ANOVA showed that in rats receiving 4-AP after CBX, the total duration of SWA episodes was significantly lower than in rats receiving 4-AP after saline (Figure 3B), and these differences were significant at p < 0.0001.
A comparative analysis of the instantaneous repetition rate of spike waves in the composition of SWA episodes was carried out in rats of experimental groups 1 and 2 (Figure 3F). Statistical analysis using the nonparametric Mann–Whitney test showed that the instantaneous repetition rate of spike waves in the composition of SWA episodes did not differ between the analyzed groups of animals (7.51 ± 0.42 versus 7.59 ± 0.43). Comparison by the method of two-way ANOVA showed that the repetition rate of spike waves in the composition of seizures was not dependent on the epoch of analysis. Thus, it was shown that intracortical microinjections of CBX 30 min before the formation of an epileptic focus did not affect the instantaneous repetition rate of spike waves.
3. Conclusions
As a result of the experiments carried out here, it can confidently say that premedication with CBX in the 4-AP model of epilepsy leads to a decrease in latency period and total seizure duration and also leads to prevention of the development of status epilepticus. Intracortical administration of CBX does not affect the instantaneous repetition rate of spike waves in seizures of epileptiform activity. The in vitro results confirm that CBX reversibly blocks epileptiform activity in living rat hippocampal slices. Despite the fact that the full mechanism of suppression of epileptic activity by CBX remains unclear, it is highly likely that the astrocytic syncytium plays a role in this mechanism, and suppression of synchronized activity is mediated through an ultrahigh concentration of extracellular potassium. It is likely that specific regulators of GJ activity in astrocytes, especially those more selective then CBX, have great potential in antiepileptic therapy and should be the subject of future studies.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the founders.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020663