Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Nanostructured lipid carriers (NLC) are lipid nanoparticles of the second generation made up of solid lipid matrices mixed with liquid lipids (oils). The hybrid system between NLC and hydrogel may results in the enhancement of each component's synergistic properties in the mechanical strength of the hydrogel and concomitantly decrease aggregation of the NLC. Therefore, the advanced hybrid component development in nanotechnology provides superior functionality in the application of scientific knowledge for the drug delivery industry.
- nanostructured lipid carriers
- hydrogel
- pharmaceutical
1. Nanostructured Lipid Carriers
Nanostructured lipid carriers are typically between 200 and 400 nm in size. The different nanosizes of NLC are determined by different preparation techniques. Long-term flocculation and creaming have been demonstrated to make the upper nanosize range > 700 nm less stable. Producing sizes smaller than 200 nm necessitates higher surfactant concentrations, which are often undesirable in formulations. However, NLC with a size of 100 nm frequently have problems because they recrystallize. However, for some applications, NLC with a size of 100 nm are of particular interest due to their superior ability to penetration into the skin. To address this, Baiseng et al. (2013) developed a method for matching the lipid phase’s required hydrophilic–lipophilic balance (HLB). NLC can strongly immobilize drugs and prevent particles from coalescing due to their solid matrix [9,10]. Consequently, the mobility of the incorporated drug molecules is greatly reduced in the solid phase. In addition, the liquid oil droplets in the solid matrix increase the drug loading capacity, while the less ordered lipid matrix allows better drug accommodation [11]. Figure 1 depicts the composition of nanostructured lipid carriers (NLC).
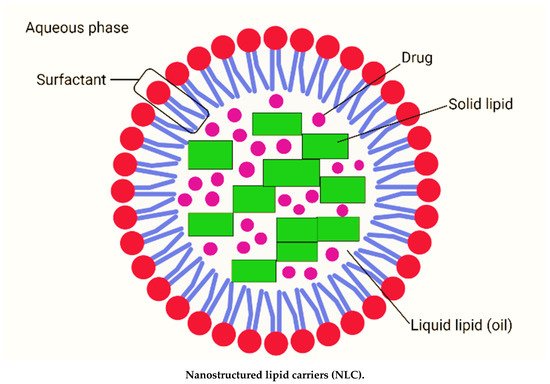
Figure 1. Composition of nanostructured lipid carriers (NLC) consisting of solid lipid, liquid lipid (oil), drug, surfactant and aqueous phase (created with biorender.com (accessed on 2 September 2021)).
1.1. Preparation of Nanostructured Lipid Carriers (NLC)
In order to prepare of NLC, various formulation approaches exist, which include high-pressure homogenization (HPH) [12,13,14], solvent emulsification-evaporation [15], phase inversion [16], high speed homogenization and/or ultrasonication [17], and solvent injection [18]. The main advantages and limitations of these preparation techniques have been discussed in Table 1.
Table 1. Preparation method of NLC with its advantages and limitations.
| Method | Advantages | Limitations | References |
|---|---|---|---|
| High pressure homogenization (HPH) |
A well-known and widely used technique. It is a simple and low-cost technique. Product with a more homogeneous particle size distribution and better overall stability. Both aqueous and non-aqueous dispersion media are employed. |
It is not possible to completely avoid drug exposure to high temperatures. Incompatible with thermolabile drugs. |
[12,13,14,19] |
| Solvent emulsification-evaporation | Large-scale production is feasible. | Uses organic solvent | [15] |
| Phase inversion | It is related to the two procedure. The inversion procedure needs three temperature cycles (85–60–85 °C). |
Cumbersome technique | [16] |
| High speed homogenization and/or ultrasonication |
Low particle size: 30–180 nm Low shear stress |
Metal shading leads to contamination Energy intensive process |
[17] |
| Solvent injection/displacement | Easy handling and fast production process Lipids are dissolved in water missicible solvent |
Use organic solvent | [18] |
1.1.1. High-Pressure Homogenization (HPH)
HPH technology has proven itself as a reliable and effective method for producing lipid nanoparticles. This approach may also be employed for large-scale manufacturing, unlike previous ones. There were two types of homogenization methods developed: hot and cold. In both methods, the pharmaceutical ingredient is dissolved or disseminated in melted lipid prior to HPH. The fluid in the homogenizer is moved by high pressure (100–2000 bar). The average particle size is sub-micron. Homogenization offers various advantages, including large-scale manufacturing, the lack of organic solvent, increased product stability, and enhanced drug loading, but it is difficult to use due to high pressure and temperature conditions [19].
1.1.2. Solvent Emulsification–Evaporation
The lipid is dissolved in a water-insoluble organic solvent in this procedure. Following that, an emulsion in an aqueous phase with surfactant is created. Evaporation under lower pressure is used to remove the solvent from the emulsion. Evaporation causes nanoparticles to disperse in the aqueous phase (using lipid precipitation process in the aqueous phase). This approach, unlike cold homogenization, will not be subjected to thermal stress; nonetheless, the organic solvent utilized in this process is a drawback. The particle size varies depending on the solid lipid and surfactant [15].
1.1.3. Phase Inversion
Transformation of an o/w type to a w/o type of emulsion is termed “phase inversion”. It can be induced by changing the temperature, and the temperature at which the inversion occurs is referred to as the PIT. This technique mainly depends on the change in the properties of polyoxyethylated surfactants at different temperatures. The hydrophilic–lipophilic balance (HLB) value of surfactants defined by Griffin is valid at 25 °C. At this temperature, the hydrophilic parts of the surface-active compounds are hydrated to a certain extent. Moreover, the dehydration of the ethoxy groups occurs when an increase in temperature. Thus, the lipophilicity of the molecules of the surface-active compounds rises with the decrease in HLB value. At a certain point, the surface-active compounds with affinity to the aqueous and lipid phase are equal—this temperature is called the phase inversion temperature. In this method, lipid, drug, water and surfactant are mixed together by magnetic stirring, and three heating and cooling cycles are performed. The mix is then diluted with cold water causing phase inversion of the emulsion and breaking, which results in the NLC [16].
1.1.4. High Speed Homogenization and/or Ultrasonication
Lipid nanoparticle dispersions are obtained by dispersing the melted lipid in the warm aqueous phase containing surfactants by high sheer homogenization followed by ultrasonication. This method primarily involves heating of a solid lipid to approximately 5–10 °C above its melting point. The lipid melt is dispersed in an aqueous surfactant solution at the same temperature under high-speed stirring to form an emulsion. Subsequent sonication reduces the droplet size of the emulsion. Gradual cooling of the warm emulsion below the crystallization temperature of the lipid yields a lipid nanoparticle dispersion. Concentrated lipid nanoparticle dispersions can be obtained by ultracentrifugation [15,17].
1.1.5. Solvent Injection/Displacement
In the solvent injection method, lipids are dissolved in a water-miscible solvent like acetone, isopropanol, and methanol water-miscible solvent mixture and quickly injected into an aqueous solution of surfactants through an injection needle. The gradual solvent diffusion out of lipid-solvent droplets into water causes reduction of droplet size and simultaneously increases lipid concentration. In addition, the diffusion of pure solvent from the lipid-solvent droplet causes local variations in the interfacial tension at droplet surface, inducing reduction of particle size of NLC [18].
1.2. Advantages and Limitations of Nanostructured Lipid Carriers
In terms of drug release, stability, and dispersion in long-term storage, NLC outperform the first generation of lipid nanoparticles, which are solid lipid nanoparticles (SLN) [7]. The unique preparation of NLC using a different blending of solid lipids with liquid lipids (oils) results in a lipid particle-matrix with a lower melting point than the original solid lipid. Nevertheless, this matrix remains solid at body temperature [11]. The solid lipid undergoes polymorphic changes in the SLN system, such as recrystallization in a low melting, less stable modification and change to a more stable modification over time. The drug can be expelled due to changes in the crystalline structure, resulting in the precipitation of large drug crystals in the water phase.
To address this issue, adding oil to a lipid can prevent the lipid from re-crystallizing in a less stable form, as demonstrated in a few studies [20,21]. As a result, no changes in modification occur in NLC over time, and thus, no drug expulsion is obtained [22]. As no sophisticated equipment is required, the preparation of NLC is significantly less expensive and cost effective. Furthermore, NLC have a high drug loading and drug release that the particle-matrix material could modulate. As a result, the release of the drug is controlled and is not limited or solely determined by size. NLC have a clear benefit in terms of low toxicity value for their market, as they could be made from orally accepted lipids and surfactants, allowing for concept development. Lipid nanoparticles could also be useful for intraocular delivery via injection. Previous research has shown that NLC particles are biodegradable and have excellent tissue tolerability when injected into chickens [23].
Furthermore, autoclaving can be used to sterilize lipid nanoparticle suspensions. During the process, the particles melt and re-crystallize. As a result, they meet the essential requirements for sterile, biodegradable, and tissue-tolerable intraocular formulations. However, this technique has some limitations. NLC require a strong dilution of the particle dispersion that often yields less than 1% particles and the need to remove organic solvent residues. The main barrier to industrial use is the possibility of obtaining very low concentrated particle suspensions. In addition, many final products, such as tablets, require excessive water to be removed [24].】
2. Nanohybrid System: Nanostructured Lipid Carrier-Hydrogel
Typically, a semi-solid vehicle is required to disperse colloidal carrier formulations to develop suitable formulations composed of nanostructured lipid carriers (NLC) for topical, dermal, and transdermal administration. Recently, NLC hydrogel formulations have been described as one of the possible semi-solid systems for drug administration via topical, dermal, and transdermal routes [84]. The process of material hybridization is an ancient practice, as reported by the ancient Egyptians [85]. These advanced hybrid materials combine each excipient’s structural, physicochemical, mechanical, and therapeutic properties in a single final form in the drug delivery system field. Hybridization allows the creation of smart formulations or pharmaceutical forms capable of interacting specifically with biological barriers such as mucosal tissues and skin [86]. Besides that, previous studies of lipid-biopolymer hydrogels for the topical sustained release of ketoprofen [87], ofloxacin [88] and resina draconis [89] exhibited optimized properties in comparison to their lipid-related drug delivery system. Figure 1 shows a structural view of a nanohybrid system.
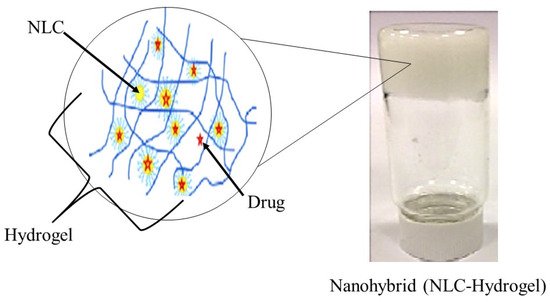
Figure 1. Structural view of nanohybrid system (NLC-Hydrogel) (created and modified with biorender.com (accessed on 2 October 2021) and adapted from [89]).
2.1. Conceptualization of NLC-Hydrogel
Three different supramolecular NLC-hydrogel designs can be proposed: (a) nano-hydrogels stabilizing single or multiple NLC, (b) NLC non-covalently immobilized in a hydrogel matrix and (c) NLC covalently immobilized in a hydrogel matrix (Figure 2).
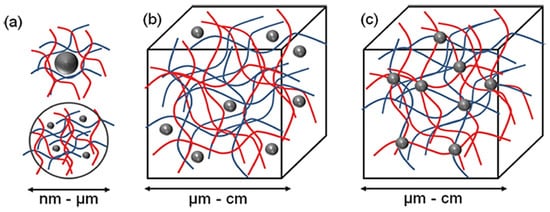
Figure 2. The concept for a combination of NLC and hydrogel to form new functional materials. Three different structural designs exist: (a) nano-sized hydrogel particles stabilizing inorganic or polymer NLC (b) NLC non-covalently immobilized in a hydrogel matrix and (c) NLC covalently immobilized in hydrogel matrix (Adapted from [1]).
According to Figure 2, the most basic method for forming an NLC-hydrogel composite is gelation of a suspension of pre-formed nanoparticles in a hydrogel-forming monomer solution. This method was used to create optically responsive optomechanical nanoparticle-hydrogel composites [90]. However, there are some downsides to this method, including the leaching of nanoparticles from the hydrogel matrix if the cross-link density is low [91]. Additionally, crosslinking groups present on the nanoparticle surface are used in the development of nanoparticle-hydrogels. The flexibility of nanoparticles as cross-linkers to form multiple bonds within gel networks (multivalency), as opposed to the two covalent bonds of a traditional hydrogel formation reaction, is a significant advantage. Furthermore, the incorporation of nanoparticles into hydrogels was shown to result in increased interfacial binding between the network and the nanoparticles, resulting in increased stiffness as well as excellent energy dissipation capability with orders of magnitude improvement in fracture resistance under compressive loading [92]. The novel combination of these two very different types of materials is expected to produce not only structural diversity but also a slew of property enhancements. Recent evidence, for example, by Liu et al. (2014), found that a silica nanoparticle-hydrogel composite made of silica nanoparticles and modified polyethylene glycol demonstrated notable improvement in tissue adhesiveness, mechanical stiffness, and bioactivity when compared to a hydrogel without nanoparticles [93]. In addition, when gold nanoparticles were immobilized in Poly-N-isopropyl amide hydrogels, significant changes in mechanical property and thermal response were also observed [94].
Tomsic et al. (2009) and Guillot et al. (2010) were the first to demonstrate the reversible incorporation of nanostructured lipid particles in hydrogels (2009) [95]. Kulkarni et al. (2011) extended the concept to the formation of dry films. Rehydration and re-dissolution of gel films could result in the recovery of nanostructured lipid particles. The size and nanostructure of lipid particles were preserved as a result of the aforementioned hysteresis [96]. Kulkarni et al. (2015) conducted one study that used a nanohybrid system of nanostructured lipid particles and polysaccharide-based hydrogel for controlled therapeutic applications for drug delivery. The nanostructured lipid particles were made by kinetically stabilizing self-assembled lipid nanostructures, and the hydrogel was made by dissolving kappa-carrageenan (KC) in water. The drug was incorporated into both native and lipid particle-loaded hydrogels, which formed thin films upon dehydration and demonstrated improved instability, as well as the ability to release more drug in an efficient manner [90].
2.2. Why Nanohybrids?
Scientists have spent decades studying nanohybrids between NLC and hydrogels. Hydrogels are now used in a wide range of biomedical applications, including drug delivery [97], wound dressing [98], and antimicrobial applications [99]. Most of these applications necessitate the use of multifunctional hydrogels and dynamic interactions with the cellular microenvironment [100]. However, one of their major limitations is their low mechanical strength, which is especially important when used for tissue engineering or other applications that require high strength, enhanced compressive and tensile properties, good elasticity, and endurance (for example, cartilage tissue) [101]. Furthermore, they are difficult to handle and use for specific applications because of their poor mechanical properties. Hydrogels research has recently shifted to optimize their chemical and mechanical properties, particularly for biomedical applications [102,103,104].
New varieties of hydrogels, including nanocomposite hydrogels, are being developed to improve the material properties of hydrogels and expand the range of their applications in medical and biotechnological fields. In addition, nanoparticles are also being applied in the consumer market. Despite their wide applications, the safety in the use of nanoparticles remains a significant challenge [105]. This obstacle can be overcome by combining them with hydrogels, resulting in lower environmental and human health risks. The hybrid combination of hydrogels and nanoparticles results in structurally diverse materials and improves their combined properties [106]. The large intermolecular spaces in the hydrogel networks serve as homes for a large number of nanoparticles and as nanoreactors for their synthesis [107]. Polymeric hydrogels act as a “host” that can accommodate various types of nanoparticles as a “guest” to form nanocomposite hydrogels [108].
The invention of nanoparticles into the hydrogel network results in the development of new materials with physical and biomedical properties that have promising applications in the biomedical field [109]. Thus, many nanocomposite hydrogels have been engineered for several biomedical applications, including drug delivery [110,111], biosensors [112], tissue engineering [113], and wound healing [114]. Past research has successfully developed an improved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulation. The lipid nanoparticles were uniformly dispersed in the gel base, retaining their spherical shape and narrow distribution. All in all, these findings demonstrated that the NLC were homogeneously incorporated into the hydrogel while maintaining the beneficial properties of NLC dispersion, particularly small particle size and homogeneity [115].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27010289
This entry is offline, you can click here to edit this entry!
