Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The application of membrane processes in various fields has now undergone accelerated developments, despite the presence of some hurdles impacting the process efficiency. Fouling is arguably the main hindrance for a wider implementation of polymeric membranes, particularly in pressure-driven membrane processes, causing higher costs of energy, operation, and maintenance. Radiation induced graft copolymerization (RIGC) is a powerful versatile technique for covalently imparting selected chemical functionalities to membranes’ surfaces, providing a potential solution to fouling problems.
- organic fouling
- pressure driven membrane processes
- polymeric membranes
- radiation induced graft copolymerization
- biofilm formation
- antifouling properties
1. Introduction
Membrane separation processes have received an ever growing interest in the past four decades, and the world has witnessed a remarkable rise in their large-scale applications in various fields, including food, water treatment, and medical and energy applications [1]. To date, membrane fouling is the major serious problem that is often encountered upon applying pressure-driven membrane processes for solid/liquid separations such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) [2]. It leads not only to a reduction in the flux and lifespan of the membrane that is coupled with an increase in the differential pressure and feed pressure, but also a reduction in the treated water quality, rise in the energy consumption and operation cost, and eventual deterrent of the widespread application of membrane technology [3]. Particularly, membrane processes such as water and wastewater treatment [4], desalination [5], dairy processing [6], fruit juice concentration [7], and whey protein concentration [8] are among those in which fouling typically poses a major drawback, impeding an efficient membrane performance.
Fouling is a complex phenomenon that occurs on membranes’ surfaces, or within their pores in liquid-based separations, because of the interactions between the foulants (e.g., bacteria, proteins, debris, and crystals) with the membranes’ rough surfaces, causing cake formation, organic adsorption, gel layer formation, inorganic precipitation, biological/microbial fouling, and full-to-partial pore blocking, all of which adversely affect the overall performance of the membrane systems [4,9]. The type and level of fouling depend on the fluid mechanics of the membrane system, the properties of the feed solution, and the characteristics of the membrane [10]. Thus, various methods have been used to reduce the membrane fouling by eliminating its promoting factors [7]. This includes pre-treatment of the feed with a disinfectant, backwash cleaning of the membrane, and optimization of the system operating conditions [11]. Other antifouling practices include modification of the membrane surface during its fabrication to endow the membrane with intrinsic resistance to fouling. This is carried out by imparting hydrophilicity, roughness, and other characteristics to the membrane surface [3]. Membrane modifications can be performed by various methods including surface coating [12], blending, which introduces bulk modification [13], combining surface modification with blending [14], chemical treatment [15], interfacial polymerization [16], graft copolymerization [17], and incorporation of inorganic metal oxides additives such as titania [18], alumina [19], zirconia [20], and silica [21] in addition to nanoparticles, carbon nanotubes [22], graphene oxide [23], and immobilization of antimicrobial additives such as silver nanoparticles [24] occur during membrane fabrication.
Graft copolymerization, one of the techniques of interest that allow the covalent incorporation of the desired chemical functionality to the membrane surface, can be carried out using chemical grafting or radiation induced graft copolymerization (RIGC). The chemical grafting is a well-established method for the surface modification of membranes, but its challenges include leaving detrimental residues, difficulty in controlling the level of grafting, and posing environmental concerns because of hazardous chemical initiators and solvents. Alternatively, RIGC, which can be carried out by low-energy (UV and plasma) and high-energy (γ-rays and electron beam (EB)) radiation, is a promising method that allows modifications of the membrane surfaces by covalent immobilization of selective antifouling moieties, with controllable levels of grafting using less hazardous chemicals [25]. Nevertheless, the application of this method has not received sufficient attention, despite its merits. The growing number of publications on the use of RIGC for polymeric membrane surface modifications in various applications during the past 20 years is shown in Figure 1. As can be seen, the number of publications is increasing exponentially, which suggests the growing interest in using the RIGC method to address the long-standing issue of membrane fouling and realize its potential.
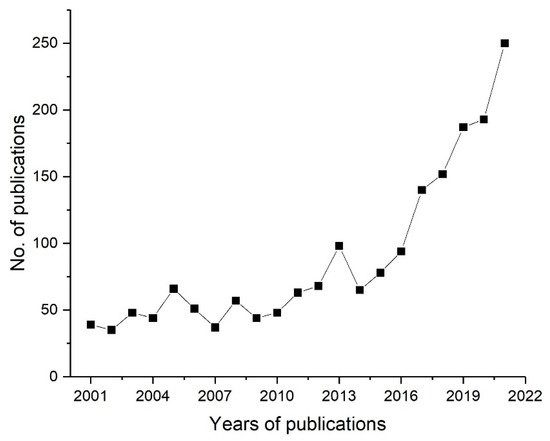
Figure 1. Number of publications on the use of RIGC for modification of polymeric membranes’ surfaces in various applications during the period of 2001–2021 (Science Direct, keyword search: radiation induced graft copolymerization, surface modification, polymeric membranes, antifouling, 5 October 2021).
Immense progressive research efforts have been made to understand the various aspects of fouling, and this progress was captured in several review articles and book chapters. This includes reviews on the fouling mechanism and key strategies for overcoming it [4,26], membrane antifouling coatings against biomolecules and protein [27,28,29,30], fouling in water treatment processes (e.g., desalination and RO) [3,4,9,31], and practiced as well as emerging eco-friendly technologies for fouling control [32]. On the other hand, few articles reviewed the progress in the antifouling modifications of the widely used membrane polymers such as poly(vinylidene fluoride) (PVDF) [33,34], the antifouling membrane surface construction using various chemistries [35], and the real-time fouling monitoring techniques [36]. However, there is a lack of information on the aspects of RIGCs as fouling mitigation methods working through membrane surface modifications with functional groups. Moreover, a review dealing with RIGC for modification of membranes using different radiation sources and the progress taking place to impart covalently attached antifouling properties is rather scarce [37].
2. Progress in Application of RIGC for Fouling Prevention
RIGC has been proposed as a very convenient and promising method for imparting antifouling characteristics to the polymeric membranes, and thus it became the subject of many investigations. The antifouling characteristics are introduced by incorporation of functional polymers that originated from various monomers such as acrylic acid (AA), acrylamide (AAm), 2-acrylamidoglycolic acid (AAG), 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS), N-(3-tert-butyl-2-hydroxy-5-methylbenzyl) acrylamide (BHMBA), 2-(dimethylamino) ethyl acrylate (DMAEA), 2-(dimethylamino) ethyl methacrylate (DMAEMA), ethylene diamine (EDA), ethylene glycol dimethacrylate (EDMA), ethylene glycol dimethyl ether (EGDME), D-gluconamidoethyl methacrylate (GAMA), glycidyl methacrylate (GMA), 2-hydroxyethyl acrylate (HEA), 2-hydroxyethyl methacrylate (HEMA), methyl acrylate (MA), methacrylic acid (MAA), methyl methacrylate (MMA), [3-(methacryloylamino)propyl]-dimethyl (3-sulfopropyl) ammonium hydroxide (MPDSAH) inner salt, N-methyl-N-vinylacetamide (MVA), N-isopropyl acrylamide (NIPAM), N-vinylacetamide (NVA), N-vinyl-caprolactam (NVC), N-vinyl formamide (NVF), N-vinyl-2-pyrrolidone (NVP), 2,4-phenylenediamine (PDA), poly(dimethylsiloxane) (PDMS), poly(ethylene glycol) methacrylate (PEGMA), poly(sulfobetaine methacrylate) (PSBMA), 3-sulfopropyl methacrylate (SPMA), sodium styrene sulfonate (SSS), and trimethylammonium (TMA) to various types of membranes for different separation processes. Membranes made of a variety of polymers such as cellulose triacetate (CTA), PA, PAN, PE, PEEK, PES, polyethylene terephthalate (PET), PP, poly(phenylene oxide) (PPO), PS, PTFE, and PVDF have been endowed with antifouling characteristics through surface modification by RIGC in many studies using different initiation techniques, the details of which are reviewed in the next section.
2.1. Membranes Modified with RIGC Using Plasma Treatment
RIGC with plasma technique has been widely used to improve the surface properties of the membranes and enhance their fouling resistance [37]. The majority of the studies applying plasma treatment for antifouling improvements were focused on the treatment of MF and UF membranes made of PES [123,124,125,126,127,128,129], PVDF [130,131,132,133], and PS [134,135,136]. Early studies reported the surface modification of PVDF [137], PE [138], and PES [124] UF membranes, which were modified by RIGC of AAm with plasma for improving hydrophilic surface properties, to overcome the fouling during protein separation. The grafted membranes exhibited better performance, marked by greater flux recoveries after cleaning, revealing reversibility of the protein fouling layer under the influence of the imparted hydrophilicity. PS and PAN UF membranes were modified by RIGC with He-plasmas, with a few monomers such as HEMA, AA, and MAA [134]. In addition, the PES UF membrane used in MBR was also modified by monomers such as AA and HEMA via RIGC with Ar-plasmas [129]. The poly(HEMA) grafted membranes demonstrated higher hydrophilicity and reduced protein fouling compared to the original membranes, and their UF performance improved in terms of both filtrate flux and bovine serum albumin (BSA) retention.
Early on, Gancarz et al. [135] investigated one of three various methods for imparting hydrophilic character to PS UF membranes by modification with AA, using RIGC initiated by plasma irradiation, as illustrated in Figure 2. The methods included introducing AA from the bulk solution, Ar plasma irradiation and grafting from the vapor phase, and plasma polymerization of the monomer vapor in a plasma reactor. The modified membrane surface had a brush-like structure with strong hydrophilicity and exhibited the most promising protein filtration properties. Two other PS UF membranes, modified by grafting of DMAEMA and AA after treatment with low-temperature plasma, were also reported [136]. The former monomer introduced positive charges that reduced the desorption of positively charged lysozyme on the modified membrane, whereas the latter imparted negative charges and reduced the adsorption of negatively charged BSA on the modified membrane.
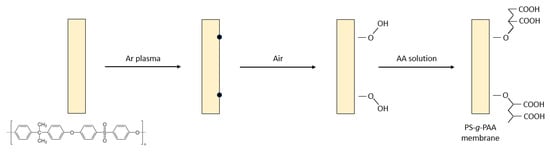
Figure 2. Modification of porous PS membrane by RIGC of AA with Ar plasma treatment.
A long-lasting hydrophilic modification was introduced to PES membranes by Ar-plasma treatment, followed by grafting of AA from a vapor phase, as reported by Wavhal and Fisher [126]. The modified membranes showed highly enhanced pure water flux and reduced protein fouling, in addition to easier recovery of the permeation flux. Meanwhile, Zhao et al. [130] used plasma pre-treatment and graft copolymerization of PVDF powder with AA to make an amphiphilic PVDF-g-PAA membrane in a one-pot process. The water flux, BSA rejection, and antifouling ability of the modified PVDF membranes were all improved because of the enhancement of wettability. Moreover, less irreversible fouling of the modified PVDF membrane was observed. The grafting of AA on polymeric membranes enhanced the antifouling properties of the membranes [127]. Positive impacts of AA grafting on the membrane hydrophilicity and antifouling properties have also been reported using other substrates such as PE [139], PTFE [140], PAN [134], and CTA [141].
Commercial PES UF membranes were treated with low-temperature He-plasmas followed by RIGC with NVP to enhance the surface hydrophilicity and roughness, as reported by Chen et al. [125]. The surface-modified membranes proved to be remarkably less prone to BSA fouling, and the recovery of permeation flux became easier compared to the pristine counterpart. In another study, Zhao et al. [142] prepared PAN NF membranes by modification with low-temperature Ar-plasma irradiation and subsequent grafting in NVP aqueous solution to improve the filtration capacity and fouling resistance. The salt rejection from a mixed salt aqueous solution of the poly(NVP) modified PAN NF membranes increased. NVP was also grafted onto PP hollow fiber membranes used in MBR for wastewater treatment. The modification was performed by RIGC with air plasma and the improvement of its limiting flux and antifouling characteristics was reported by Yu et al. [143]. The poly(NVP) modified membranes showed an enhanced filtration behavior in MBR compared to the pristine membrane. Moreover, the relative flux ratio increased by 79% and the flux recovery increased by 53%. The flux was, however, 17.9% lower than that of the pristine membrane. Overall, the modified PP hollow fiber membranes possessed excellent antifouling characteristics. The grafting of NVP on different substrates via RIGC with plasma treatment has enhanced the separation performance and antifouling properties of the membranes for various membrane processes.
Poźniak et al. [144] studied surface modification of PPO UF membranes with sulfonation (monopolar) and combined sulfonation and amine (bipolar) plasma treatment to enhance the hydrophilicity and antifouling properties. The membrane was modified by introducing sulfonic acid groups into membranes using plasma-initiated surface RIGC of SSS in comparison with chemical sulfonation (with H2ClSO3) of PPO. The bipolar amphoteric membranes with the combined sulfonic acid and allylamine demonstrated not only enhanced filtration capacities but also performed very well in the micellar-enhanced UF of mixtures of the 2,4-D herbicide and hexadecyltrimethylammonium, with 90% removal of 2,4-D herbicide from the water. In another study, Li et al. [131] attempted the surface modification of porous PVDF membrane using glycidyl methacrylate-iminodiacetic acid (GMA-IDA) containing carboxylic acid (-COOH) and tertiary amine (-N=) to prepare a bipolar membrane by RIGC using plasma treatment for the conversion of salt and water into acid and base by ED. The ionic groups were subsequently introduced to the grafted membrane by treatment with HCl and NaOH solutions, respectively. After the GMA-IDA monomer was grafted onto the surface of the PVDF membrane, the hydrophilicity of the membrane was dramatically increased. Moreover, the membrane’s ability to separate monovalent and divalent ions was enhanced, and fouling within the ED system was reduced. Earlier, the same authors prepared a similar bipolar membrane based on a porous PVDF film that was grafted with AA and DMAEA, using RIGC with plasma treatment [133]. Introduction of anionic and cationic polyelectrolytes to the anion-exchange and cation-exchange layers of the bipolar membranes by RIGC with plasma treatment has improved the membrane hydrophilicity and antifouling properties for the electrochemical processes.
A study by Khongnakorn et al. [141] clearly reported the use of LPP treatment to graft AA onto a commercial CTA membrane to improve water flux and impart anti-protein fouling properties in FO for protein recovery. The surface hydrophilicity of CTA membrane grafted with AA in the presence of CO2 and the counterpart grafted with AA in the presence of Ar was the highest at the optimum plasma exposure time of 10 s, based on the contact angle results (Figure 3a). The obtained membranes also showed a higher surface roughness, represented by the root-mean square (RMS), as depicted in Figure 3b [145]. The membrane modified with AA in the presence of Ar also demonstrated a significant improvement in the ability to maintain higher water flux over the course of the filtration experiments when compared to the untreated membrane [146]. In a BSA filtration experiment, the untreated membrane suffered the highest flux decline with 55% loss, followed by the membrane grafted with AA in of CO2 (50% loss) and that grafted with AA in Ar (36% loss) in the first cycle, as shown in Figure 3c. The flux recovery was achieved after washing with deionized water in the second cycle and chemical treatment in the third cycle. Hence, this study proved that LPP treatment followed by monomer grafting is highly effective for improving membrane anti-protein fouling performance. However, it must be mentioned that LPP treatment is impractical for most industrial applications because it requires a vacuum system, which limits the sample size. Besides, this approach lacks scalability, has high maintenance costs, and system integration is difficult [147].
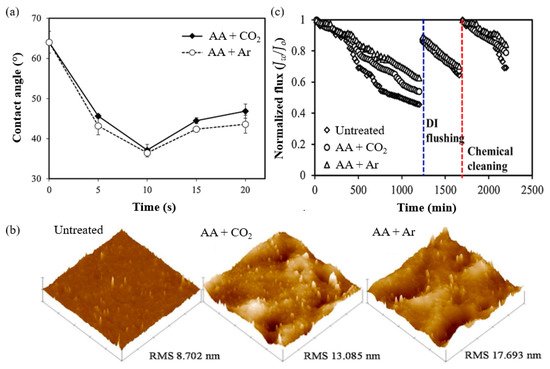
Figure 3. (a) Water contact angle, (b) 3D AFM images of surface topography, and (c) flux decline in BSA filtration. Reprinted from [141] with permission from Elsevier.
As an alternative to LPP treatment, APP treatment has been employed for fouling prevention in various applications [45,123,147,148,149,150,151]. APP treatment receives merits over LPP treatment because it can combine UV as an ion bombardment without the need to be in a vacuum space, producing high concentrations of ions and radicals, beside the ability to control the properties of grafted polymer phase [152]. Gu et al. [108] reported that after reacting with oxygen in the air, radicals created on the PES membrane surface by APP treatment were converted into peroxides and could act as initiators for graft copolymerization [108]. As the APP treatment time increased, the number of peroxides increased to the highest amount (4.5 nmol/cm2), which is comparable to that obtained using the LPP treatment [153]. The wettability of the membrane was greatly improved by APP treatment, but its antifouling capacity was not significantly improved over time. The possible explanation is that APP treatment failed to maintain the desired surface antifouling properties due to surface restructuring [123]. Extensive APP activation could be used to improve surface hydrophilicity, but this would most likely result in the membrane’s dense skin layer deterioration. Polyethylene glycol (PEG) grafted PES membrane displayed a significantly better protein resistance and antifouling performance than the unmodified and APP treated pristine PES membranes [123]. RIGC using APP treatment has also been applied to modify RO membranes to prevent mineral scaling. Kim et al. [45] utilized a two-step approach whereby a PA TFC membrane was first irradiated with APP, followed by graft copolymerization of MAA monomer. The study showed that the onset time for gypsum scaling on the PA-g-poly(MAA) TFC membrane’s surface was delayed, indicating the reduced susceptibility towards mineral scaling compared to a commercial RO membrane. It can be concluded that with optimal APP activation, the RIGC was found to improve the performance of membranes by increasing water flux and minimizing organic fouling or inorganic scaling, depending on the membrane processes and applications.
Chang et al. [154] attempted the application of RIGC using plasma treatment on an expanded polytetrafluoroethylene (ePTFE) MF membrane for biofouling prevention. Particularly, a hydrogel-like layer of PEGMA was immobilized on a H2 plasma irradiated ePTFE MF membrane. As the grafting degree of the copolymerized PEGMA increases, the hydrophilicity of the surface of the ePTFE MF membranes increases, forming a surface hydrogel-like layer in an aqueous solution with regulated coverage. The membrane with a low grafting yield exhibited a relative reduction in protein adsorption coupled with a remarkable suppression of platelet adhesion and hemocompatibility. In another study by Dong et al. [155], the PA and polyester membranes modified with PEG via RIGC using plasma treatment showed a substantial reduction in biofouling that was caused by a pathogenic bacteria, Listeria monocytogenes. The grafting of hydrophilic monomers via RIGC using plasma treatment has been proven to prevent biofouling.
Among the current research trends in curbing biofouling in membrane, processes is grafting polymeric membranes with pseudo-zwitterionic functionalities using different techniques [128,132,156,157,158]. Venault et al. [132] reported the use of glow dielectric barrier discharge plasma for inducing the surface grafting of PVDF membranes with two monomers, namely TMA and SBMA. Efficient grafting could not be achieved with SPMA, but it was successful with a combination of SPMA and TMA. The modified PVDF membranes reduced the adsorption of BSA and lysozyme and resisted the attachment of Escherichia coli, and thus they were considered very effective in reducing biofouling in static conditions compared to pristine PVDF membrane. High resistance to blood cells and low hemolysis activity showed that pseudo-zwitterionic membranes are compatible with human blood. Apart from that, Jhong et al. [158] prepared ePTFE membranes grafted with zwitterionic PSBMA and PEGMA via plasma-induced RIGC. The ePTFE-g-poly(PSBMA) membrane exhibited high wettability and became less adhesive towards protein, human blood, tissue cells, and bacteria. The preparation of membranes with high hemocompatibility and biocompatibility and low biofouling by RIGC with zwitterionic monomers using plasma treatment could be very beneficial for hemodialysis application.
An example of modifying a polymeric membrane surface with zwitterionic monomer by RIGC using air plasma (corona) treatment for flux enhancement and fouling reduction was reported recently by Salimi et al. [128]. SPMA was grafted onto the surface of a PES membrane, leading to desired grafting yields by controlling grafting conditions. The grafted membrane displayed a maximum increase of about 1100% in permeate flux for oil/water emulsion filtration compared to the unmodified membrane. Moreover, the pure water flux increased by up to 1000%, together with a maximum flux recovery ratio enhancement of 180%, which is a significant improvement. Despite its scarcity, the use of corona treatment followed by grafting of monomer has been reported to yield less damage to membrane bulk and pore structure compared with other plasma treatments and, most importantly, enhance the antifouling properties of the membranes [127,128,129,139].
RIGC using plasma treatment has plenty to offer, whereby its impact on the improved antifouling properties of polymeric membranes is significant for various membrane processes. When compared to non-modified membranes, the modified membranes attained lower fluxes but with higher flux recoveries. Furthermore, this method needs a very short time to modify the membrane surface. The type of plasma process, whether it is LPP or APP, and the selection of plasma gas, which controls the grafting yield, are among the parameters that made this modification method effective. The previous studies, which addressed various modifications of polymeric membranes via RIGC using plasma treatment for fouling prevention, are summarized in Table 1.
Table 1. Summary of previous studies on the application of RIGC using plasma treatment for fouling prevention.
| Substrate | Grafted Monomer | Main Finding(s) | Refs |
|---|---|---|---|
| CTA | AA | Art was more effective than CO2 to increase water flux and decrease reverse salt flux and fouling tendency | [141] |
| PE | AAm | Membranes of different functional groups with opposite surface charges can be utilized for covalent immobilization of protein | [138] |
| PE | AA | The modified membrane showed a significant increase in hydrophilicity, water flux, and BSA solution flux | [139] |
| PES | PEG, amines, zwitterionic compounds | High stability of PEG polymer chain and protein-resistance of PEG grafted PES were achieved compared to using UV as radiation source | [123] |
| PES | AAm | Due to the improved surface hydrophilicity, the grafted membrane was less susceptible to BSA protein adsorption and had higher flux recoveries after cleaning | [124] |
| PES | NVP | BSA fouling was significantly reduced, and the cleaning of modified membranes was easier to recover permeation flux | [125] |
| PES | AA | Modified membranes were hydrophilic, less prone to protein fouling, and had a higher pure water flux | [126] |
| PES | AA | The grafting of AA occurred on the membrane surface and on the pore walls inside the membranes, which enhanced the fluxes and the antifouling properties of the membranes | [127] |
| PES | SPMA | Outstanding water–oil flux by the modified membranes was achieved at grafting temperature of 65 °C and grafting yield of 0.489 mg/cm2, followed by flux recovery of 87.5% | [128] |
| PES | AA, HEMA | The membrane modified with HEMA has reduced fouling propensity due to the absence of deep pockets in its structure, whereas PES-g-PAA had a damaged membrane structure | [129] |
| PS | AA | Grafting in solution resulted in hydrophobic membranes with significantly smaller pore sizes. When grafting in the vapor phase, AA grafted surface layer closely resembled pure PAA, which was hydrophilic in a basic environment | [135] |
| PS | DMAEMA, AA | The adsorption of lysozyme on the DMAEMA grafted membrane was greatly reduced. AA grafted membrane, which exhibited a stronger negative surface charge, has caused reduction in BSA adsorption due to the increased electrostatic repulsive force | [136] |
| PS, PAN | HEMA, AA, MAA | After grafting with HEMA, the water contact angles of PAN and PSf reduced. These membranes also had significantly less fouling and better protein UF performance | [134] |
| PAN | NVP | With an increase in both graft reaction time and grafting medium temperature, water flux decreased significantly | [142] |
| PPO | SSS | Micellar-enhanced UF of mixtures of the 2,4-D herbicide and hexadecyltrimethylammonium bromide was much better with bipolar amphoteric membranes with combined sulfonic acid and allylamine | [144] |
| PP | EGDME | Polyethylene oxide-like PP with antiplatelet behavior was formed using EGDME as monomer, with almost no sign of platelet adhesion and accumulation | [147] |
| PP | NVP | Flux recovery, flux reduction, and relative flux ratio were 53% higher, 17.9% lower, and 79% higher, respectively, than the neat PP membrane | [143] |
| PA TFC | MAA | The optimal membrane surface’s onset time for gypsum scaling was delayed by a factor of 2–5, hence reduced the propensity for mineral scaling | [45] |
| PA, polyester | PEG | The PA-g-PEG and polyester-g-PEG membranes had similar hydrophilicity, and they showed 96% reduction in biofouling caused by Listeria monocytogenes | [155] |
| PVDF | AA | Due to the presence of PVDF-g-PAA, less irreversible fouling was detected in the modified PVDF membrane | [130] |
| PVDF | GMA-IDA | Surface hydrophilicity of the PVDF-GMA-ID bipolar membrane was increased | [131] |
| PVDF | TMA, SPMA | In static conditions, BSA and lysozyme adsorption tests, as well as an Escherichia coli attachment test, revealed the reduced biofouling by pseudo-zwitterionic PVDF membranes | [132] |
| PVDF | AA and DMAEA | The contact angle of bipolar membranes decreased | [133] |
| ePTFE | AA | The grafting of AA onto ePTFE resulted in highly hydrophilic membranes with high water uptake | [140] |
| ePTFE | PEGMA | The surface hydrophilicity of PEGMA grafted ePTFE membranes increased, which reduced protein adsorption and platelet adhesion | [154] |
| ePTFE | PSBMA, PEGMA | The zwitterionic PSBMA grafted ePTFE membrane had the best non-bio adhesive character against biomacromolecules and cells | [158] |
2.2. Membranes Modified with RIGC Using UV Treatment
RIGC using UV treatment is suitable for modification of intrinsically photoactive polymeric membranes. This approach requires either a photo-sensitive base polymer, such as PES and PS, or the introduction of photo-sensitive groups onto the membrane surfaces prior to graft copolymerization, and involves the direct generation of free radicals from polymer substrate under UV irradiation [83].
The earlier work by Pieracci et al. [159] reported modification of PES UF membranes by RIGC with NVP, NVF, and NVC using UV irradiation to obtain hydrophilic membranes with low fouling surfaces. The membrane modified with poly(NVP) showed a 25% increase in hydrophilicity, a 49% decrease in BSA fouling, and a 25% increase in BSA retention, compared to untreated PES membrane. Moreover, this membrane attained the best combination of low fouling and high flux among all tested membranes. UV-assisted RIGC of NVP onto 50 kDa PES UF membranes was also investigated, using two different techniques involving dip modification and immersion modification, by the authors [160]. The grafted PES membranes showed highly wettable surfaces with superior fouling resistance compared to the pristine membrane. The membranes grafted by all the above modification techniques were prone to the simultaneous loss of BSA rejection and permeability, and the level of such loss depended on the grafting level. This was due to the pore obstruction caused by invaded grafted polymer chains, and such effect was predominant at high poly(NVP) concentrations, suggesting that, as radiation cleaved PES bonds and enlarged the pores, a high density of long chains was created on the surface. The wettability and fouling data indicated that the irreversible adsorptive fouling can be eliminated by modifying the base membrane with poly(NVP) at a low grafting yield, but with a negative impact on the permeability. On the other hand, a higher grafting yield causes a further loss in permeability. Pieracci et al. [161] extended their work to further improve the permeability of the poly(NVP) grafted PES UF membrane, using 2-mercaptoethanol as a chain transfer agent followed by ethanol cleaning. The increased concentration of chain transfer agents reduced the graft chain density and length. Meanwhile, the non-grafted homopolymer that initially blocked the membrane pores was removed by ethanol due to membrane swelling. Consequently, the permeability of the PES UF membrane improved, but at the expense of losing protein rejection. It was suggested that the incorporated poly(NVP) grafts promote the swelling and pore enlargement of the membrane, hence causing severe loss of protein rejection. On the contrary, the incorporation of poly(NVP) grafts on PES and sulfonated PES NF membranes via RIGC using UV treatment by Kilduff et al. [162] significantly reduced fouling caused by natural organic compounds, while maintaining pure water permeability and solute rejection at the levels comparable to those found in neat PES NF membranes.
A porous PP MF membrane prepared by UV-assisted RIGC of NVP showed lower protein adsorption and platelet adhesion and more hemocompatibility with the increased grafting yield [163]. PP MF membranes were also modified by RIGC with NVP using UV radiation under various reaction conditions, as illustrated in Figure 3. The increase in the monomer concentration (10–70 vol%) and treatment time showed minor effects on the grafting yield of poly(NVP) on PP MF membrane. It was suggested that UV irradiation was limited for RIGC initiation of NVP on the membrane surface [163]. In another study, PP membrane with novel antibacterial properties comprising surface-immobilized poly(NVP)-iodine complex was also reported by Xing et al. [164]. The NVP was grafted onto PP membranes using RIGC with UV irradiation followed by complexation of iodine on poly(NVP) grafted membrane. The content of iodine could be adjusted by controlling grafting, which could be manipulated by varying the irradiation time or the monomer concentration. The obtained membrane was proven to have efficient antibacterial activity against Escherichia coli, Staphylococcus aureus, and Candida albicans.
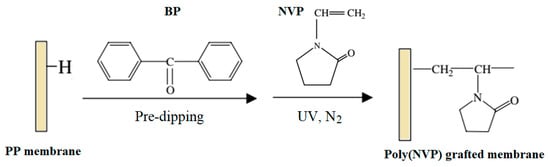
Figure 3. Modification of porous PP membrane by RIGC of NVP with UV irradiation. Reprinted from [163] with permission from Elsevier.
PP membranes were also modified with other hydrophilic monomers using the same RIGC method for fouling prevention [165,166,167,168]. For example, Gu et al. [165] modified PP microporous membranes with sugar-containing monomers, such as GAMA. The antifouling properties of the membrane improved dramatically during the MBR as the grafting chain length was increased. The highly hydrated poly(GAMA) layer grafted on the membrane surface prevented the foulant adhesion and, as a result, the foulant could be easily removed by water washing, imparting this membrane with reversible fouling characteristics. In another study, Hu et al. [166] used HEMA to improve the surface hydrophilicity of a PP microporous membrane with the addition of FeCl3 and BP. The former acted as an inhibitor for homopolymerization, whereas the latter was a photo-initiator. This increased grafting yield and imparted a remarkable enhancement of the hydrophilicity of the membrane, leading to better antifouling and hemocompatibility than the neat PP membrane. Apart from that, grafting of AAm on PP membranes via RIGC with UV treatment also improved the hydrophilicity and flux recovery of the PP-g-poly(AAm) membranes [167,168].
Other polyolefin membranes, such as PE membranes, were also modified, whereby hydrophilic monomers such as 2-methacryloyloxyethyl phosphorylcholine (MPC), NVP, AAm, and methacryloyl poly(ethylene glycol) (MPEG) were graft-copolymerized onto PE membrane using UV irradiation [164]. Of all monomers, the incorporation of poly(MPC) was found to significantly improve protein adsorption together with the platelet adhesion resistance, and so the NVP grafted membranes were compared to pristine PE membranes. Surprisingly, membranes incorporated with poly(AAm) and poly(MPEG) did not show any effect on protein adsorption. PE membranes that are hydrophilic, electrically neutral, and have smooth surfaces are less likely to be fouled [169].
Selecting the right monomers for surface modification of polymer substrates via RIGC using UV treatment is important to achieve desired membrane properties. In several attempts to prepare membranes with low biofouling properties, hydrophilic monomers such as quaternized 2-(dimethylamino) ethyl methacrylate (qDMAEMA), AMPS, and HEMA possessing respective basic, acidic, and neutral natures were used to modify PES and PVDF membranes [170,171,172]. The membranes modified with qDMAEMA were found to have distinct antibacterial surface properties in a way that they stopped microorganisms’ growth, reducing the formation of fouling biofilms. The membranes showed a strong antimicrobial effect against Escherichia coli, and this biocidal property enhanced the resistance to biofouling in water treatment applications. In another study, MPC was grafted on a PEEK membrane surface via RIGC using UV treatment to prepare membranes with a high wettability and low protein adsorption for medical applications [173]. Taniguchi et al. [174] tested six different hydrophilic monomers: NVP, HEMA, AA, AAG, SPMA, and AMPS for modification of PES UF membranes with RIGC using UV treatment for natural organic matter removal. The AA grafted membrane displayed the most stable filtration over a long period and recorded the lowest fouling with zero irreversible fouling. Evaluation of membranes grafted with these monomers for BSA rejection suggested that AA was the most sensitive to UV oxidation and copolymerization. Thus, AA has been mostly selected to modify polymeric membranes by RIGC, using UV treatment for various membrane processes [175,176,177]. A small increase in the wettability of AA grafted membrane was enough to prevent irreversible fouling, whereas the high swelling imposed by NVP and HEMA caused reduced BSA rejection, despite the improvement in reducing fouling compared to the commercial PES UF membrane [178]. Grafting of AA for fouling prevention showed superiority compared to the grafting of amino monomers (AAm, EDA, PDA) or other acrylic monomers (HEMA, PEGMA) when using different polymer substrates such as PAN [179], PP [168], and PVDF [180] membranes. However, there was also a study that reported the higher flux recovery ratio of PEGMA grafted PES nanoporous membranes compared to grafting with AA, PDA, and EDA monomers [181], whereas another study on polyimide (PI) UF membranes showed otherwise [182]. In a comparison study between PEG and three monomer pairs (PEG–NVF, PEG–NVA, and PEG–MVA) for modification of PES via RIGC using UV treatment, PEG–NVA was the best monomer pair grafted on the PES membrane to resist fouling by BSA, proving that binary systems can improve the protein resistance of a membrane [183].
Helin et al. [184] grafted a PS UF membrane with MA by RIGC using UV treatment. The increase in the grafting yield improved the hydrophilicity of grafted membranes, and the results of pure water and BSA solution permeation proved that adding MA to the PS structure improves the membrane’s antifouling property. Similar outcomes were reported by Yu et al. [185], who incorporated a zwitterionic molecule, MPDSAH on a PS UF membrane. The hydrophilicity of the membrane was improved while showing superior separation and consistent pure water flux. The MPDSAH grafted membranes outperformed the PS UF membrane in terms of antifouling properties in the pH range of 4.5–10.0. In contrast, a PES UF membrane modified by RIGC with PEGMA using UV treatment outperformed the membranes modified by new generation material, zwitterionic N,N-dimethyl-N-(2-methacryloyloxyethyl-N-(3-sulfopropyl)ammonium betaine (MMESPAB), in terms of fouling resistance [186].
In a recent study to develop an anti-coagulation PET membrane surface for blood filtration [187], a photoactive pseudo-zwitterionic copolymer (PZC) made up of 2-carboxyethyl acrylate, trimethyl-2-methacroyloxyethylammonium chloride, and 2-methacryloyloxyethyl-4-azidobenzamide was first synthesized by free radical polymerization under Ar atmosphere. A PET substrate was then immersed in the PZC solution, followed by irradiation with UV to produce PZC grafted PET membrane. The presence of copolymerized PZC in the fibrous membrane structure with a balanced composition of cationic and anionic groups resulted in excellent anti-coagulation surfaces. The PZC grafted PET membrane, prepared by RIGC using UV treatment, was able to prevent platelet adhesion and activation. Weinman et al. [188] first developed a novel zwitterionic polymer, namely poly(2-((2-hydroxy-3-(methacryloyloxy)propyl)dimethylammonio)acetate)(poly(CBOH)), to modify PES UF membrane via UV-induced graft copolymerization for reduced biofouling. The modified membrane had a unique property whereby its surface chemistry may switch between the anti-fouling, zwitterion mode and an anti-microbial, quaternary amine mode by changing the pH. The preparation of membranes with low biofouling can be achieved by RIGC with zwitterionic polymers using UV treatment.
In a study by Mondal and Wickramasinghe [189], a commercial PA TFC membrane was grafted with NIPAM using RIGC with UV irradiation to prepare an antifouling NF membrane with a relatively high salt rejection. BP was initially adsorbed onto the membrane surface by immersion in its ethanol solution followed by grafting with NIPAM. The modified membrane attained a significant hydrophilicity increase because of the formation of poly(NIPAM) hydrogel layers that vary in density depending on reaction parameters. Despite experiencing reduced water flux, the formation of a thick hydrogel mat on the membrane grafted surface resulted in a huge increase in salt rejection and fouling resistance compared with the commercial NF membrane. Moreover, the grafted membrane surface could release the accumulated foulants on the membrane surface when flushed with warm water, thanks to the temperature responsive characteristic of poly(NIPAM) [190,191]. According to this study, changing the membrane’s surface chemistry by RIGC of poly(NIPAM) using UV treatment has significantly reduced the negative effects of colloidal fouling in NF.
Huang et al. [99] fabricated a PEEK UF membrane, with a surface modified by HEA as a hydrophilic monomer through UV induced graft copolymerization under various reaction conditions, to control not only the grafting yield and surface hydrophilicity but also surface morphology and fouling resistance. Particularly, the monomer concentration and irradiation time were used to control the grafting yield, contact angle (hydrophilicity), and fouling, as shown in Figure 4.
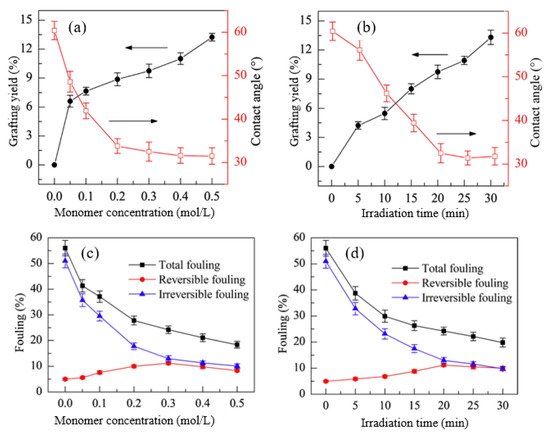
Figure 4. The effects of HEA concentration (irradiation time = 20 min) on: (a) grafting yield and contact angle and (c) fouling and the effects of irradiation time (HEA concentration = 0.3 mol/L) on (b) grafting yield and contact angle and (d) fouling. Reprinted from [99] with permission from Elsevier.
Figure 4a shows the impact of monomer concentration and UV irradiation time on grafting yield and surface hydrophilicity. The increase in both HEA concentration and reaction time caused an increase in the grafting yield, which consequently increased the hydrophilicity, as indicated by the reduction in the contact angle. This suggests that the change in the properties of modified membranes is a function of grafting yield which is heavily dependent upon reaction conditions. A similar trend was also reported in a study by Zhang et al. [192], in which NVP was grafted on a PVDF/PES MF membrane using UV irradiation under various monomer concentrations and reaction times. The improved hydrophilic property due to increased monomer concentration and irradiation time has gradually reduced the total fouling and irreversible fouling caused by hydrophobic molecules, as indicated in Figure 4b, which shows the small increase in reversible fouling after grafting of HEA [193]. This proves that the hydrophilic HEA chains help to weaken the membrane-BSA interaction during cleaning, thus allowing the flux to increase. These findings show that increasing grafting density and graft chain length can improve antifouling performance, which is consistent with the previous research. [194,195]. Prolonging the irradiation time produces more active sites and increases graft initiations, and thus increases the graft chain number [185]. However, furthering irradiation time to a longer extent can lead to excessively long graft chains, causing membrane pore blocking and permeability reduction. In another study, Susanto et al. [196] prepared low fouling UF membranes made up of PES that were modified by PEGMA through RIGC with UV irradiation. The results revealed that the most important parameter for adjusting the degree of functionalization was UV irradiation time, followed by monomer concentration. Although the membranes showed higher fouling resistance and rejection, pore constriction or even blocking by grafted poly(PEGMA) has resulted in a decrease in permeability. Therefore, it can be concluded that the increase in the grafting density endows a rise in the hydrophilicity and improves the membrane permeability, unlike the growth in the grafted chain length, which leads to pore blockage and reduction in the membrane permeability [197].
Xueli et al. [198] prepared BHMBA grafted PS UF membrane using RIGC UV irradiation in the presence of BP. Despite the addition of BP, the grafting yield of the grafted membrane only increased by 24% without significant changes in the surface roughness, antifouling property, and antibacterial efficiency compared to those of the membrane grafted in the absence of BP. One plausible reason is that the grafting took place only on the upper membrane surface, not on the membrane pores [199]. The main disadvantage of this approach is the dependence on BP concentration; the low concentration of BP at the membrane surface leads to a low grafting efficiency, whereas a high bulk BP concentration in the monomer solution can lead to homopolymerization. Thus, optimization of the concentration of photoinitiator concentration together with other reaction parameters is essential for effective membrane modification by RIGC with UV irradiation. The various modifications reported for polymeric membranes via RIGC using UV irradiation for fouling prevention are summarized in Table 2.
Table 2. Summary of previous studies on the application of RIGC using UV treatment for fouling prevention.
| Substrate | Grafted Monomer | Main Finding(s) | Ref. |
|---|---|---|---|
| PEEK | HEA | Due to the increased surface hydrophilicity, the irreversible fouling of optimized membrane decreased from 51% to 10.9% | [99] |
| PEEK | MPC | The membrane displayed high wettability and high anti-protein adsorption | [173] |
| PVDF | AA, HEMA, PDA, EDA | Antifouling properties such as flux recovery and fouling resistance of modified membranes were improved | [180] |
| PVDF/PES | NVP | Grafted membrane showed good fouling resistance due to the decreased BSA adsorption, reduced fouling degree by 66%, and better flux recovery by 32% after chemical cleaning | [192] |
| PA TFC | NIPAM | Change in surface chemistry of grafted membrane due to formation of temperature responsive poly(NIPAM) hydrogel improved fouling resistance and salt rejection | [189] |
| PES | NVP, HEMA, AA, AAG, SPMA, AMPS | NVP, AMPS, and AA-modified membranes had high protein retention, high solution flux, and low irreversible fouling | [174,178] |
| PES | AA, HEMA, PDA, EDA | The membranes suffered a decrease in permeation of pure water and milk water but with improved protein rejection. PES membrane grafted with poly(HEMA) had the best antifouling properties | [181] |
| PES | PEG, NVF, NVA, MVA | PES membrane grafted with PEG–NVA binary monomer pair displayed the best BSA fouling resistance | [183] |
| PES | AA | Modified MF membranes had lower permeability but showed 100% flux recovery after cleaning, following the filtration of Escherichia coli | [175] |
| PES | AA | Modified NF membranes exhibited higher flux, higher humic acid rejection, and lower irreversible fouling | [176] |
| PES | AA, AAm | The separation ability and flux recovery ratio of PES-g-AAm surpassed PES-g-AA and unmodified PES membranes | [177] |
| PES | PEGMA | Modified membrane with high monomer concentrations (40 g/L) and medium irradiation times (1.5–3 min) demonstrated greater flux, fouling resistance, and higher protein rejection | [196] |
| PES | PEGMA, MMESPAB | PEGMA and MMESPAB grafted PES membranes displayed far better adsorptive fouling resistance than unmodified PES membrane | [186] |
| PES | NVP, NVF, NVC | In comparison to the initial membrane, modified membranes showed higher fluxes and less BSA fouling, especially for PES-g-poly(NVP) membrane | [159] |
| PES | NVP | Membranes irradiated for 60 s had a lower fouling tendency. However, under long irradiation times, the pore structure increased in size, increasing membrane fouling | [162] |
| PES | NVP | Both the dip and immersion modification techniques produced membranes with increased wettability and reduced irreversible adsorptive fouling | [160] |
| PES | NVP, 2-mercaptoethanol | The permeability of the membranes decreased as the grafting yield increased | [161] |
| PES | Poly(CBOH) | The membrane had a switchable feature between the anti-fouling, zwitterion mode and an anti-microbial, quaternary amine mode by adjusting the pH | [188] |
| PES, PVDF | AMPS, qDMAEMA, HEMA | HEMA was less susceptible to fouling on the neutral hydrophilic membrane surface than on the charged membranes | [172] |
| PES, PVDF | qDMAEMA, AMPS | Modified membranes were more biofouling resistant. The number of proliferated bacterial cells from countable colonies was much lower for qDMAEMA grafted membranes | [170,171] |
| PS | MA | Hydrophilicity of graft copolymer membrane increased as the MA grafting yield increased. The antifouling property of the membrane was improved | [184] |
| PS | BHMBA | Modified membranes had low surface roughness which corresponded to the improved antibiofouling property and excellent antibacterial properties against Escherichia coli | [198] |
| PS | MPDSAH | As the grafting yield increased, the modified membranes’ hydrophilicity and antifouling properties improved | [185] |
| PAN | AA, HEMA, PEGMA | Adsorption and fouling were reduced for both negatively and positively charged membranes | [179] |
| PP | AA, AAm | The modified membranes performed better in the MBR than the unmodified ones, with the AA grafted membrane having the best antifouling properties | [168] |
| PP | GAMA | After 70 h of continuous operation in the MBR, the modified membranes had reduced water flux of up to 87.2%, at increased length of the grafted chains | [165] |
| PP | HEMA | Because of the increased surface hydrophilicity, the modified membrane demonstrated improved protein resistance and hemocompatibility | [166] |
| PP | AAm | The inner part of the membrane had a higher grafting yield than the outer part. The modified membrane had better flux recovery of approximately 70% | [167] |
| PP | NVP | The surface hydrophilicity increased with the increase in grafting yield. The amounts of adsorbed BSA and adhered platelets on membrane decreased substantially | [163] |
| PP | NVP | The membrane with iodine complex has a desirable antibacterial property against Escherichia coli, Staphylococcus aureus, and Candida albicans | [164] |
| PE | PDMS, PEG | The membrane showed reduced fouling towards Pseudomonas aeruginosa as the membrane surface became smoother and more hydrophilic, with decreased membrane charge | [169] |
| PE | MPC, NVP, AAm, MPEG | The poly(MPC) and polyvinylpyrrolidone (PVP) grafts on PE membrane substantially helped to reduce the plasma protein adsorption and the platelet adhesion | [164] |
| PI | AA, HEMA, PDA | Pure water and milk water permeation of PI membranes decreased while the protein and salt rejection increased after grafting, especially with PDA monomer | [182] |
| PET | PZC | The effectiveness of PZC in preventing membrane blockage and filtering platelets from blood plasma helped the grafted membrane to exhibit superior anti-biofouling properties | [187] |
2.3. Membranes Modified with RIGC Using γ-rays
Compared to UV and plasma treatments, γ-rays have a high penetration depth and strong energy that can produce radicals at the inner parts of the polymeric materials, regardless of their thickness. Due to this advantage, γ-rays are suitable for preparation of pore-filled membranes, integrating the mechanical strength of the substrate with the high conductivity of polymer electrolyte for ion exchange applications, such as fuel cell [200], ED [201], and RED [202]. Nevertheless, several studies considered the utilization of this graft copolymerization technique for pressure-driven membrane processes, which makes this technique, as applied to fouling prevention, appealing [203,204,205,206,207,208,209,210,211,212,213]. The early study by Shim et al. [209] reported the modification of the surface of porous PP membranes by RIGC of HEMA using γ-rays. The grafting yield reached up to 76% by optimizing the absorbed dose and reaction time. The modified membranes acquired increased hydrophilicity and showed a decrease in the water flux due to the narrowed and plugged pores with grafted poly(HEMA). The rise in hydrophilicity resulted in a maximum two-fold increase in BSA solution flux. After deionized water and chemical cleaning, the flux recovery of the modified membranes was superior to that of the unmodified membrane. It was deduced that the grafted poly(HEMA) chains prevented the hydrophobic interactions between BSA molecules and the membrane surface and reduced BSA adsorption. As a result, the modified membrane acquired improved antifouling and washing properties.
In another study, Sehgal and Rattan [205] studied the RIGC of MMA onto irradiated isotactic PP membrane via peroxidation method, starting with γ-rays’ irradiation from a Co60 source to fabricate a PP-g-poly(MMA) membrane. The reaction conditions were manipulated to promote graft copolymerization over homopolymerization, which is very possible because of the monomer’s high reactivity. The latter was suppressed by the addition of a small amount of FeCl3 as an inhibitor. The obtained membrane acquired good hydrophilicity and remarkable swelling behavior in different solvents. A similar isotactic PP membrane grafted with NVP using the same RIGC method was also reported by the same authors, who obtained membranes with improved hydrophilicity and pH sensitivity [214]. NVP was also grafted on a PP MF membrane using RIGC with γ-rays and UV radiation. The pre-irradiation with γ-rays was proven to be more efficient with respect to grafting yield. The observed reduction in the protein adsorption and platelet adhesion provided evidence for the enhancement of and hydrophilicity and hemocompatibility by incorporation of poly(NVP) chains to the PP membrane. The water flux increased at a low grafting yield, reaching a maximum of 7.3 times higher than the pristine membrane. A higher grafting yield undermined the water flux, which was a result of competition between the hydrophilicity and the variation in pore size of the grafted membrane [163].
Deng et al. [213] investigated the application of RIGC of MAA on PES powder using γ-rays to prepare PES MF membranes by polymer solution casting. The membrane attained increasing hydrophilicity as a function of grafting yield. Consequently, the swelling was increased and pore size was enlarged, leading to an improved filtration flux. The pH value of the aqueous solution had no effect on the properties of the MF membranes made from neat PES. On the contrary, the MF membranes fabricated from the poly(MAA) grafted PES powder was pH-dependent. The increased degree of swelling decreased both pore size and flux, which were attributed to the increase in the ionization of the grafted poly(MAA) side chains with increasing alkalinity of the solution. A similar PES membrane, but in a hollow fiber form with hemocompatibility properties, was also investigated by Wang et al. [206]. The membrane was prepared by RIGC of SSS, AA, and NVP on PES substrate using γ-rays. Interestingly, there was no platelet adhesion observed on the PES-g-poly(AA/NVP/SSS) membrane. Furthermore, the amounts of BSA and bovine fibrinogen (BFG) deposited on the membranes showed a remarkable decrease for PES-g-poly(AA/NVP/SSS) membrane compared to PES-g-PVP membrane. In addition, the modified membranes did not cause hemolysis or activate complement, and the blood clotting time was slightly delayed. These results confirm the effective endowment of the anti-platelet adhesion property and hemocompatibility to PES membranes, which became more suitable for hemodialysis application.
One of the advantages of RIGC using γ-rays is that it allows bulk grafting or modification of the membrane core, and therefore dramatic changes in the physico-chemical properties, such as hydrophilicity and wettability of the whole membrane, can be introduced. To discuss the impacts of such changes on fouling prevention, a study was carried out by Shen et al. [215] to modify PVDF membrane by RIGC with HEA using γ-rays, and the properties of the prepared PVDF-g-poly(HEA) membrane were evaluated. The copolymerization of HEA on the PVDF membrane produced a membrane with a rougher surface and higher hydrophilicity compared to the control membrane, as indicated by the AFM images and water contact angle results shown in Figure 5a–c. Furthermore, the water content ratio of the grafted membrane was three times higher than that of the control membrane (Figure 5d), indicating that the grafted membrane has significantly improved in its wettability. The substantial increase in membrane wettability has proven that RIGC produces a homogeneous membrane structure with grafted hydrophilic polymer chains across the membrane.
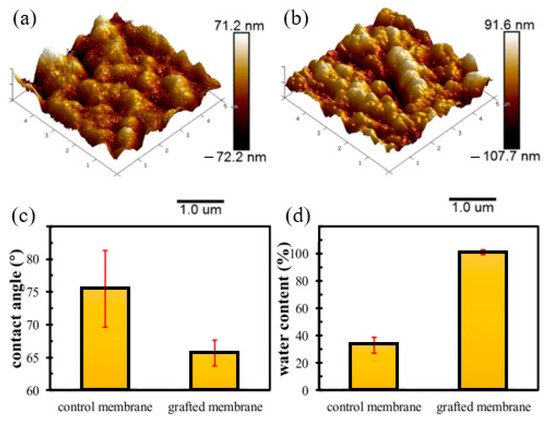
Figure 5. AFM images, contact angle, and water content of the control and grafted membranes: (a) AFM image of the control membrane; (b) AFM image of the grafted membrane; (c) contact angle; (d) water content. Reprinted from [215], published by Nature.
The grafted chain matrix on the membrane had a higher tendency to experience configurational change or swelling due to the chemical structure of the grafted membrane that absorbs more water molecules [195]. Swelling of the chain matrix which can be affected by either pH or ionic strength of the solution would reduce the pore size, leading to regulation of the water flux [215,216]. It was found that the flux of the grafted membrane has a strong dependence on pH in the acid-stage filtration. At a neutral pH, the grafted HEA chains tend to swell because the chemical potential of the entire matrix is the lowest in this state. Considering the solution’s ionic strength, high ionic strength results in high electric double layer compression, which helps to resist swelling of the grafted chain matrix.
The impact of this adjustable pore size on the antifouling property of the grafted membrane is shown in Figure 6. The grafted membrane exhibited a lower water flux compared to the control membrane, caused by the swelling of the grafted chain matrix in pure water without ionic strength, resulting in a significant reduction in the pore size. When the pure water was replaced by BSA solution with the same pH value of 7.0, the flux of the control membrane decreased significantly. The flux profile versus filtration time shows that the control membrane experienced severe fouling after only a short period of filtration. In contrast, the PVDF-g-poly(HEA) membrane demonstrated a greater value of BSA solution flux than that of pure water in three complete filtration cycles. The BSA solution’s relatively high ionic strength prevented the swelling of the grafted chain matrix and enlarged the membrane’s surface pore size, and thus, the BSA flux was found to be greater than the water flux of the grafted membrane. The flux’s decreasing rate demonstrated unequivocally that the grafted membrane had greater antifouling ability.
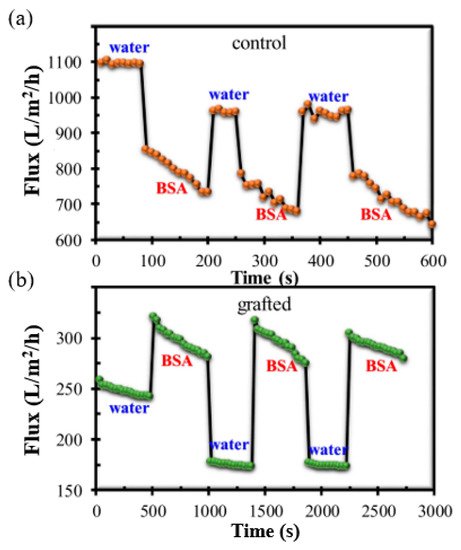
Figure 6. Antifouling performance of membrane by alternate filtration of pure water and BSA solution: (a) flux of control membrane and (b) flux of grafted membrane. Reprinted from [215], published by Nature.
To further improve the antifouling ability of PVDF membranes, a new strategy for grafting HEA through γ-rays radiation was proposed by Shen et al. [212] by conducting thermodynamic analyses. The grafted membrane possessed an improved antifouling ability for filtration of sodium alginate solution, which was experimentally evidenced by the increased flux recovery ratio and the decrease in the irreversible fouling ratio. The results suggested that the improved antifouling performance was primarily due to improved hydrophilicity and reduced strength of thermodynamic interactions between the grafted membrane and foulants.
Other hydrophilic monomers such NVP [203,208], polyvinyl alcohol (PVA) [211], and NIPAM [204] were also grafted on PVDF membranes for fouling prevention. In the preparation of PVDF-g-poly(PVA), the PVA was directly anchored onto PVDF membrane surfaces via RIGC with γ-rays irradiation [211]. The modified PVDF membrane attained high hydrophilicity and underwater superoleophobicity, which improved the anti-oil fouling and cleaning properties of the membranes. Qin et al. [203] prepared PVDF MF membrane by modification with NVP using RIGC with γ-rays. The grafting yield increased as the NVP concentration, absorbed dose, or dose rate increased. This caused an increase in both surface hydrophilicity and surface roughness coupled with a decrease in the pore size. Furthermore, the membrane’s water uptake and water flux were also improved. It was proven that the modified membranes showed better antifouling properties. Similar trends were observed in a study by Yu et al. [204], whereby an amphiphilic copolymer, PVDF-g-poly(NIPAM), was synthesized via RIGC using γ-rays, followed by phase inversion to produce membranes (Figure 7). The membrane water flux increased drastically when the concentration of the grafted copolymer chains increased [204]. This trend was caused by the pore-forming ability of the amphiphilic additive that enhanced the membrane hydrophilicity.
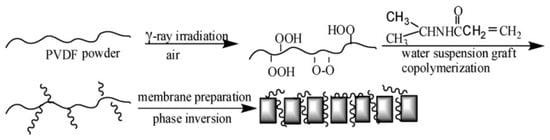
Figure 7. Modification of PVDF powder by RIGC of NIPAM with UV irradiation, followed by membrane preparation by phase inversion. Reprinted from [204] with permission from Elsevier.
The recent work by Li et al. [210] highlighted the modification of PA TFC RO membranes by PVA via coupling of interfacial polymerization with RIGC using γ-rays. The incorporation of poly(PVA) grafts improved the salt rejection of the RO membrane by increasing the degree of crosslinking of the separation layer, hence strengthening the steric hindrance. Consequently, the prepared membrane acquired a salt rejection efficiency of 99.4%. This was accompanied by an improvement in surface hydrophilicity. In addition, poly(PVA) grafts gave the membrane excellent antifouling properties, as demonstrated by the BSA fouling test and the reduced irreversible flux decline ratio.
Thus, it can be concluded RIGC using γ-rays is rather unique compared to the use of plasma and UV treatments, in the sense that it allows bulk grafting depending on irradiation dose rate and absorbed dose. Therefore, the number of studies reporting the use of γ-rays for the modification of polymeric membranes via RIGC for fouling prevention has increased in the past decade. A summary of previous studies is presented in Table 3.
Table 3. Summary of previous studies on the application of RIGC using γ-rays for fouling prevention.
| Substrate | Grafted Monomer | Main Finding(s) | Ref. |
|---|---|---|---|
| PES | SSS, AA, NVP | All modified membranes’ contact angles, protein adsorption, and platelet adhesion decreased. The modified membranes had good hemocompatibility | [206] |
| PES | MAA | Membrane prepared from PES-g-poly(MAA) powder exhibited the flux of acid solution up to four times that of basic solution | [213] |
| PVDF | HEA | The grafted membrane had lower pure water flux than the control membrane but showed noticeably higher BSA solution flux than the pure water flux | [212,215] |
| PVDF | NVP | As the grafting yield increased, the contact angle decreased, and water uptake, RMS, water flux, pore size, and water flux recovery of the membrane increased | [203] |
| PVDF | PVA | The modified membrane achieved oil rejection up to 99.5%. The oil fouling on modified PVDF membranes was almost reversible, with flux recovery of 98% | [211] |
| PVDF | NVP | Maximum grafting yield of 17.7% was obtained when reaction was carried out in water for 3 h at a monomer concentration of 20% (v/v) and an absorbed dose of 40 kGy | [208] |
| PVDF | NIPAM | The increased amount of PVDF-g-poly(NIPAM) in membrane enhanced its hydrophilicity and heightened the water flux | [204] |
| PP | HEMA | With increased grafting yield, the modified membranes’ contact angle decreased. The modified membrane had a higher solution flux, lower BSA adsorption, and better flux recovery | [209] |
| PP | MMA | Maximum grafting yield of 85% was obtained at 25 kGy radiation dose, 0.04 wt% inhibitor concentration, 6 wt% monomer concentration, 60 °C reaction temperature, and 120 min reaction time | [205] |
| PP | NVP | The amounts of adsorbed BSA and adhered platelets on membrane decreased substantially | [163] |
| PP | NVP | The increased roughness of the grafted membrane surface was due to the formation of grafted chains on the polymer surface | [214] |
| PA TFC | PVA | The surface hydrophilicity of the PVA grafted RO membrane was significantly increased and the membrane had excellent antifouling property | [210] |
2.4. Membranes Modified with RIGC Using EB
RIGC using EB is a simple and very fast technique that allows surface as well as bulk grafting depending on the acceleration energy of electrons and, thus, it is a distinctive facility for the modification of membranes and films [150,217] During the early application of RIGC with EB for fouling prevention of polymeric membranes, a binary mixture of SSS and AA was grafted to improve the hydrophilicity of PVDF and PTFE membranes [218,219]. Particularly, Liu et al. [218] modified PVDF membranes with high-energy EB using pre-irradiation (under vacuum), followed by a single step grafting with a mixture of AA and SSS to introduce a hydrophilic character to the membrane. This was evident from the reduced water contact angle of the modified PVDF compared to the pristine membrane.
In another study, Xi et al. [219] introduced the hydrophilic sulfonate groups by a single-step grafting method with binary monomer solution of AA and SSS on PTFE porous membranes, pre-irradiated by EB under vacuum. The grafting yield was found to be dependent upon the irradiation dose and the AA content in the binary monomer, as shown in Figure 8. The presence of AA made it possible to graft SSS, which is difficult to graft due to its strongly hydrophilic nature [206,218,219]. Figure 8b shows that, as the AA content in the binary monomer increases, the grafting yield increases. The grafted membrane acquired a strongly hydrophilic character unlike the strongly hydrophobic neat PTFE counterpart.
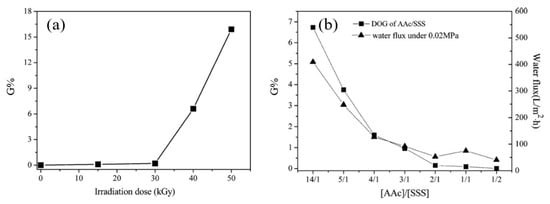
Figure 8. Effect of reaction conditions on the grafting yield: (a) effect of irradiation dose and (b) effect of AA content in binary monomer mixture [AA/SSS]. Reprinted from [219] with permission from Elsevier.
In another study by Liu et al. [220], grafting of PEGMA onto PVDF surface by RIGC using EB was conducted, whereby the factors affecting the grafting yield were investigated. The rise in the concentration of PEGMA monomer led to a rapid increase in the grafting yield, which was affected by solution pH. The high pH made PEGMA hydrophilic, increased viscosity of the solution, and became rather incompatible with the hydrophobic membrane surface, which inhibited monomer diffusion onto the PVDF membrane surface and slowed the grafting process [221]. In contrast, the PEGMA monomer had a hydrophobic structure at lower pH, which made it more compatible with the hydrophobic PVDF membrane, leading to rapid monomer diffusion and an increase in the grafting yield. The maximum grafting yield was 21%, obtained at pH 1.0 of the monomer solution. RMS decreased from 5.64 to 1.84 nm by grafting due to the presence of the grafted poly(PEGMA) brushes, according to 3D AFM images of surface topography shown in Figure 9a. Grafting also affected the hydrophilicity of the membranes, as shown in Figure 9b, whereby the contact angles of the grafted membrane decreased significantly in the presence of poly(PEGMA) brushes. The hydrophilic nature of the poly(PEGMA) side chains significantly improved the hydrophilicity of PVDF membranes.
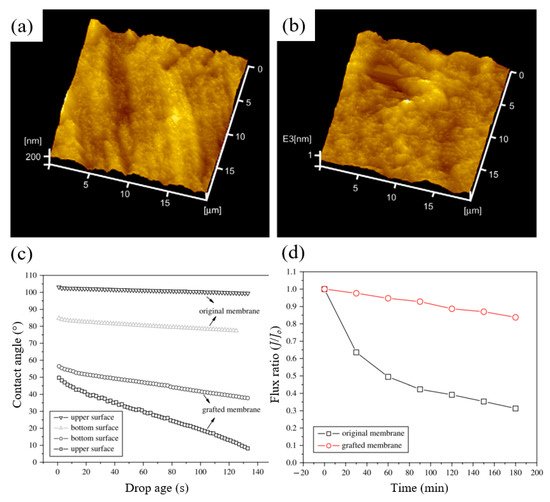
Figure 9. 3D AFM images of surface topography of: (a) original membrane; (b) grafted membrane; (c) water contact angle and (d) flux ratio of 1.0 g/L BSA solution to the initial pure water flux. Reprinted from [220] with permission from Elsevier.
The effect of grafting on the degree of fouling is shown in Figure 9c. In 3 h, the flux of the original membrane dropped to about 30% of the initial pure water flux, while the flux of the grafted membrane had maintained more than 85% of the initial pure water flux. The grafted hydrophilic poly(PEGMA) chains weakened the hydrophobic interactions between BSA molecules and the PVDF membrane, thus affecting BSA surface adsorption.
The application of RIGC using EB was also performed on sulphone-based polymer substrates for fouling prevention. Schulze et al. [222] modified the surface of PES membranes by immersing in the solutions of the low-molecular weight monomers with different hydrophilic functionalities: carboxylic, sulfonic, and phosphoric acids, amines, alcohols, and zwitterionic compounds, followed by EB treatment. Results showed that the modified membranes experienced a substantial reduction in albumin and myoglobin adsorption. In another study, a simultaneous RIGC with EB was employed to prepare PS NF membranes with high negative charge density for chromium ion, Cr(VI), and removal [223]. AMPS monomer was grafted on both the outer and inner surfaces of a PS substrate. The Na2SO4 rejection was improved at the expense of losing permeate flux as the negative charge density increased, due to the smaller mean pore size of the membrane. Cr(VI) was successfully removed from an alkaline aqueous solution by the modified NF membrane. With a permeate flux of 23.8 Lm−2h−1 at 4 bar and pH 9.0, the membrane grafted under 80 kGy with 10% AMPS solution showed 95.1% rejection of Cr(VI). Interestingly, the presence of Na2SO4 and NaCl in the experimental range posed no effect on Cr(VI) removal performance, thus reducing the propensity for inorganic scaling problems.
By applying RIGC by EB, grafting of hydrophilic polymers, which include PEG, pluronic (PLU), PVA, PVP, polyallylamine hydrochloride (PAH), and polystyrene sulfonate (PSS), was performed on PVDF membranes for biomedical applications [224]. The modified PVDF membranes showed better wettability compared to the pristine counterpart. Based on contact angle results, grafting of PEG, PLU, and PSS yielded high hydrophilicity. In addition, the irradiated membranes did not cause hemolysis or coagulation when incubated with whole blood. Overall, PVP modification produced the best results in terms of membrane stability due to the decrease in contact angle and blood coagulation. Schulze et al. [217] also covalently attached a digestive enzyme, trypsin, to the surface of a PVDF MF membrane by a one-step RIGC using EB. The biocatalytic membranes showed significantly improved antifouling properties compared to the neat PVDF MF membrane. Moreover, trypsin could be controlled by changing pH to actively degrade a fouling layer of proteins. The modified membrane restored 90% of the initial water permeation flux, whereas the neat membrane recovered only 27%. It can be concluded that the RIGC using EB did not produce any toxic breakdown products or long-term reactivity, hence ensuring its safe utilization for biomedical applications.
In a study by Nguyen et al. [225], a commercial PVDF/PVP membrane was irradiated at 10 and 100 kGy with EB in the presence of zwitterion L-cysteine, phosphocholine, and DMAEMA to enhance the fouling resistance of the membrane. A smoother surface and smaller pore sizes were achieved in the modified membranes with the zwitterion compound, accompanied by the improved antifouling capacity exhibited by the lower flux decline and prominent flux recovery. Compared to the neat and irradiated PVDF/PVP membranes with a 10 kGy absorbed dose, the irradiated PVDF/PVP membranes with a 100 kGy absorbed dose showed lower initial fluxes. The 10 kGy irradiated PVDF/PVP membrane displayed the best fouling resistance in the presence of L-cysteine.
Shawky et al. [226] reported the modification of poly(vinylidene fluoride)-co-hexafluoropropylene (PVDF-co-HFP) NF membrane by incorporation of the povidone-iodine complex. This was carried out with RIGC of NVP using EB and subsequent I2 immobilization. The obtained membrane showed excellent antimicrobial activity in the form of a complete inhibition (killing, not filtering) of the bacterial growth against Escherichia coli and Staphylococcus aureus, compared to the pristine and (PVDF-co-HFP)-g-PVP membranes. The I2 immobilized membrane also demonstrated remarkable improvement in pure water permeation flux and bacterial filtration through the membranes, indicating the reduced biofouling.
Recently, Lim and Shin [25] modified PVDF membranes via RIGC using EB with GMA/EDMA binary monomer followed by a sulfonation process. The grafted PVDF membranes with EDMA produced a denser structure, which reduced the number of oxirane groups converted to sulfonic groups. The modified membrane demonstrated the highest ion exchange capacity (1.61 meq/g) when grafted with 0.5 w/w% GMA in the absence of EDMA. This membrane showed the highest level of hydrophilicity among other modified membranes. In terms of filtration, the negatively charged membranes had higher water permeability because of their electrostatic repulsion and sieving effect. The electrostatic repulsion between the membrane and foulants also helped to reduce membrane fouling. It was found that the use of binary monomer in this study is unnecessary, as the presence of EDMA only limits the modification of the PVDF-g-poly(GMA) membrane.
Despite its merits, the RIGC technique using EB has not been fully utilized for fouling prevention until recently. This is likely caused by the captivity of most of the EB accelerators in radiation research institutes or relevant industries, where they are dedicated to routine production. Because of the speed and the absence of any hazardous material during surface modification and the high stability of the grafted membrane, this method is thought to be suitable for the modification of polymeric membranes, not only for typical pressure-driven membrane processes but also for medical applications such as hemodialysis, with improved membrane hemocompatibility and biocompatibility. The modification of polymeric membranes via RIGC using EB for fouling prevention is summarized in Table 4.
Table 4. Summary of previous studies on the application of RIGC using EB for fouling prevention.
| Substrate | Grafted Monomer | Main Finding(s) | Ref. |
|---|---|---|---|
| PES | Carboxylic, sulfonic and phosphoric acids, amines, alcohols, zwitterionic compounds | This modification resulted in significantly reduced protein adsorption at the membrane surface with selected functional molecules | [222] |
| PS | AMPS | The grafted membranes achieved NF performance at a relatively large pore size due to the high negative charge density resulting from the high grafting yield | [223] |
| PTFE | AA, SSS | AA/SSS binary monomers had a synergistic effect on grafting yield and membrane hydrophilicity with increase in AA content and irradiation dose | [219] |
| PVDF | AA, SSS | The surface hydrophilicity of the grafted membrane improved significantly | [218] |
| PVDF | PEGMA | Immobilizing hydrophilic comb-like poly(PEGMA) brushes on the PVDF membrane surface enhanced both hydrophilicity and fouling resistance | [220] |
| PVDF | PEG, PLU, PVA, PVP, PAH, PSS | Improved membrane wettability was indicated by lower water contact angles. Hemocompatibility tests revealed no unwanted hemolysis, and hydrophilic polymers were found to reduce blood coagulation | [224] |
| PVDF | Trypsin | The modified membrane had significantly improved antifouling properties. The fouling layer formed on the membrane’s surface can be actively degraded during filtration, restoring the membrane’s original permeability | [217] |
| PVDF/PVP | L-cysteine, phosphocholine, DMAEMA | Membranes exposed to absorbed dose of 10 kGy had higher permeate flux and lower cake resistance. The membrane irradiated with 10 kGy in the presence of L-cysteine had the best long-term antifouling capacity | [225] |
| PVDF-co-HFP | NVP | The PVP grafts on the membrane was capable of hosting I2, thus imparting a very strong antimicrobial activity to the membrane, which further lessened the biofouling | [226] |
| PVDF | GMA, EDMA | Addition of EDMA only resulted in a denser membrane structure and reduced the amount of oxirane groups converted to sulfonic groups. The PVDF-g-poly(GMA) membrane had a higher ion exchange capacity and improved hydrophilicity with electrostatic effect | [25] |
2.5. Membranes Modified with RIGC Containing Metal Nanoparticles
The use of nanoparticles to change the surface properties of membranes with respect to hydrophilicity and charge for imparting antifouling functions to the obtained nanocomposite membranes has been widely investigated [227]. Various metals such as Ag and Cu [228] and metal oxides such as Fe2O3, CuO, TiO2, and ZnO [229] have been used for the development of membranes with antimicrobial characteristics. Modification of membranes via in situ formation of Ag nanoparticles has been performed through covalent coating [230] to eliminate the leaching factor that defeats the expected performance of the membranes [231]. In situ synthesis of metal nanoparticles on polymeric surfaces and membranes aided with radiation was also reported elsewhere [232,233,234], whereby the membranes attained an enhancement in the hydrophilicity and antifouling properties. The introduction of a hydrophilic layer onto a membrane surface via thermal grafting [235] and atom transfer radical polymerization [236], followed by the loading of nanoparticles, has attracted considerable attention due to the advantage of long-term stability.
The metal ions can be imparted to polymer functional groups introduced to the membranes by RIGC via complexation (chelation) from a metal salt solution, which allows non-destructive membrane functionalization. The subsequent reduction in the complexed metal ions leads to the formation of metal nanoparticles covalently bonded to the membrane, adding another significant step towards the development of reactive membranes with biofouling resistance [230]. The reduction reaction of the metal ions in the salt form or metal precursors was carried out either by direct reduction through H radicals that were formed during irradiation [237] or by chemical reduction using a reducing agent [238]. The in situ synthesis of nanoparticles can be performed during or after RIGC. The concept of using RIGC to produce metal nanoparticles is illustrated in Figure 16, whereby, in the early study of Ping et al. [239], Ag nanoparticles were successfully synthesized via chemical reduction of Ag+ complexed on the membrane surface by NABH4 for improving the antibacterial activity. The grafted PAA acted as stabilizers in the reactions by allowing the retention of Ag nanoparticles between the chains in the graft [237].
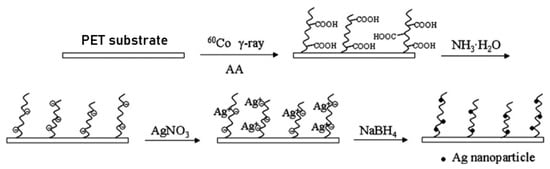
Figure 16. Modification of PET substrate by RIGC of AA using γ-rays with the in-situ formation of Ag nanoparticles. Reprinted from [239] with permission from Elsevier.
In a study by Sawada et al. [238], a PES hollow fiber membrane containing Ag nanoparticles was prepared by RIGC of AAm with UV irradiation in the presence of BP, followed by complexation with AgNO3 and subsequent reduction by NABH4 [238]. The obtained composite membrane containing Ag nanoparticles in a poly(AAm) gel layer exhibited high potential for applications requiring both organic antifouling and antibacterial properties [238]. Further work by He et al. [240] also reported the loading of Ag nanoparticles on a PES membrane in the same manner, following the UV induced graft copolymerization of the hydrophilic PSBMA, and the membrane showed improved antibacterial properties and biocompatibility.
Another method to load the nanoparticles onto the membranes is by adding pre-synthesized nanoparticles into the monomer solution during the grafting process. El-Arnaouty et al. [207] grafted NIPAM and incorporated ZnO nanoparticles onto PA TFC RO membranes via RIGC, using γ-rays for fouling prevention. The incorporation of NIPAM and ZnO nanoparticles onto PA TFC membranes significantly improved both anti-biofouling and chlorine resistance properties of the commercial PA RO membrane. Other pre-synthesized nanomaterials, such as TiO2 nanoparticles [241] and multi-walled carbon nanotubes [242,243], were also introduced to the membranes by RIGC.
This entry is adapted from the peer-reviewed paper 10.3390/polym14010197
This entry is offline, you can click here to edit this entry!
