Inspired by nature, significant research efforts have been made to discover the diverse range of biomaterials for various biomedical applications such as drug development, disease diagnosis, biomedical testing, therapy, etc. Polymers as bioinspired materials with extreme wettable properties, such as superhydrophilic and superhydrophobic surfaces, have received considerable interest in the past due to their multiple applications in anti-fogging, anti-icing, self-cleaning, oil–water separation, biosensing, and effective transportation of water. Apart from the numerous technological applications for extreme wetting and self-cleaning products, recently, super-wettable surfaces based on polymeric materials have also emerged as excellent candidates in studying biological processes.
- nature
- polymeric materials
- super-wettable surfaces
- bioinspired material
- biomedicine
1. Extreme Wetting Surfaces Inspired by Nature and Their Applications in Biomedicine
1.1. Cell Configuration for Studying Cellular Interactions
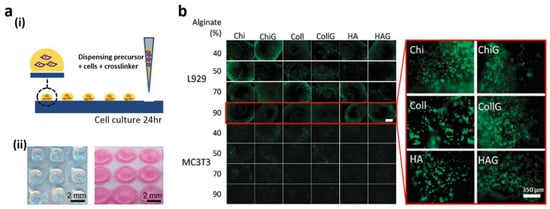
1.2. Functional 3D Cell Spheroids
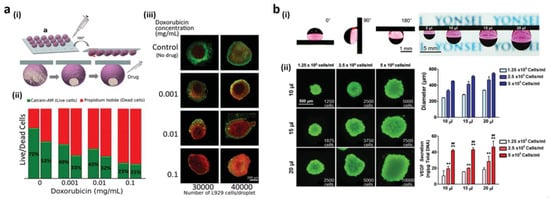
1.3. Biomedical Devices
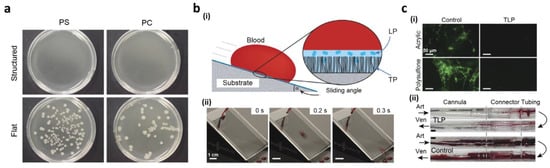
1.4. Lab-on-a-Chip Based on Open Channel Droplets
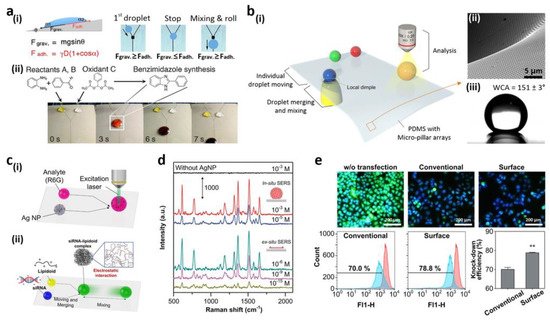
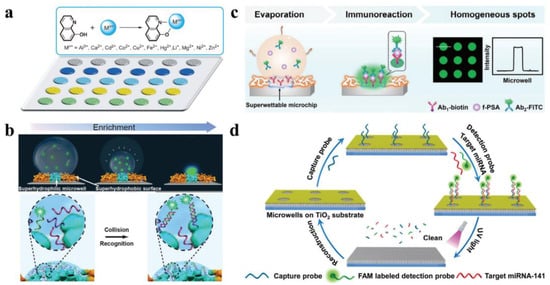
1.5. Fluorescent Intelligence Sensing Based on Bioinspired Super-Wettable Patterns
| Polymeric Materials | Fabrication Method | Application | Wetting Property | Reference |
|---|---|---|---|---|
| Hyaluronic acid (HA) | Phase separation | Tissue engineering | Hydrophobic | Ref [27] |
| Polyethylene (PE) | Shrink-induced mold method |
Antibacterial | Superhydrophobic | Ref [29] |
| Polycarbonate (PC) | Shrink-induced mold method |
Antibacterial | Superhydrophobic | Ref [29] |
| Polystyrene (PS) | Electrohydrodynamics (EHD) | Tissue engineering | Superhydrophobic | Ref [71] |
| 1H,1H,2H,2H-perfluoro-decyl trichlorosilane (PFDTS) | Wet chemical self-assembly |
Conductive stainless steel | Superhydrophobic | Ref [72] |
| Poly(vinyl alcohol) (PVA) | Solvent evaporation | Petal effects | Hydrophilic-Superhydrophobic | Ref [74] |
| Poly(p-xylylene) (PPX) | Soft lithography | Self-cleaning | Superhydrophobic | Ref [91] |
| Polydimethyl siloxane (PDMS) | Soft lithography | Water adhesion | Hydrophilic-Superhydrophobic | Ref [91] |
| Polydopamine (PD) | Soft lithography | Water adhesion | Hydrophilic-Superhydrophobic | Ref [91] |
| Polytetrafluoroethylene (PTFE) | Sol-gel strategy | Water adhesion | Superhydrophobic | Ref [176] |
| Polyvinylpyrrolidone (PVP) | Sol-gel strategy | Self-cleaning | Superhydrophobic | Ref [176] |
| Polyamide (PA) | Dip-coating method | Water and oil separation | Superhydrophobic | Ref [181] |
| Silane-modified polymer (SMP) | Spray-coating method | Water and oil separation | Superhydrophobic | Ref [181] |
This entry is adapted from the peer-reviewed paper 10.3390/polym14020238
