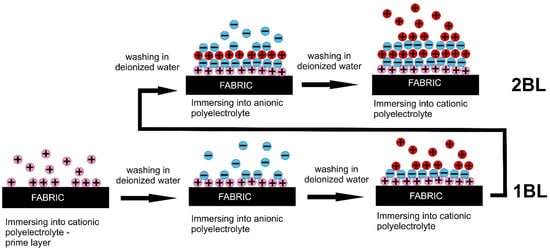
Layer-by-layer deposition is a simple technique, able to effectively deposit active compounds on textiles. The major drawback of this technique is the poor interfacial adhesion between the textile material and polyelectrolyte solution, which depends, generally, on the hydrophilicity and surface charge of the substrate, the polyelectrolyte charge, pH and ionic strength of the solution. The general process of LbL deposition on textiles is shown in Figure 1. Before LbL deposition, the surface of textile materials should be charged enough, either positively or negatively, depending on the charge of polyelectrolytes used in the process.
Cellulose fibers are highly reactive due to the presence of surface hydroxyl groups, which enable them to react with FR compounds forming semi-durable or durable FR finishes. Polyester and polyamide fibers have a limited number of functional groups (such as -OH, -COOH, -O-CH
2-CH
2-, -NH
2, etc.) on the fiber surface, which reduces the FR processability of the fabrics [
69,
70,
71]. Even though cotton is composed of more than 90% cellulose, a negligible amount of surface-located hydrophobic waxes, pectins and proteins make raw cotton quite hydrophobic. Therefore, commercial wet processing, such as desizing to remove size, scouring to clean hydrophobic waxes, pectins and proteins and bleaching to increase the whiteness of cotton, is conducted to render cotton hydrophilic and able to accept aqueous treatment. Since wet processing of cotton is economically unfavorable due to production of effluents with high chemical oxygen demand (COD) and biochemical oxygen demand (BOD), enzymatic scouring is an excellent method to overcome these negative issues by treating the cotton at lower temperatures, for less than one hour, in near-neutral pH conditions [
72,
73].
2. Layer-by-Layer Deposition to Reduce Flammability of Cotton
The majority of scientific papers on the application of LbL deposition to reduce the flammability of fabrics has been devoted to cotton. The compounds used for the deposition of cotton are long-chain organic polymers [
85,
86,
87,
88,
89] and short-chain organic molecules [
90,
91,
92], as well as suspensions of inorganic nanoparticles [
93,
94,
95].
One of the first studies regarding the LbL deposition of cotton with FRs was performed in 2010 by Li et al. They treated cotton fabrics with 5–20 BLs of cationic branched polyethyleneimine (BPEI) of different pH and anionic sodium montmorillonite (MMT) clay of different concentration. The study revealed that the layers became thicker by increasing the pH of the BPEI solution or the concentration of MMT clay. Cotton treated with 20 BLs of BPEI at pH 7 and 1 wt% MMT showed reduced afterglow time in vertical flame tests [
85].
Polyethyleneimine (PEI) is a highly charged cationic polymer, rich in nitrogen, that exists in linear and branched states. The difference between these two polymers is in the type of amino group; linear PEI possesses primary and secondary amino groups, whereas branched PEI also possesses tertiary amino groups [
96]. As a nitrogen rich and positively charged polymer, PEI is an excellent candidate for LbL deposition on a negatively charged, chemically bleached cotton surface. MMT is nanoclay consisting of an aluminum oxide/hydroxide layer stuck between silicate layers. Most of the clay minerals tend to have a negative charge resulting from the substitution of the silica cation (Si
4+) by the aluminum cation (Al
3+) in the clay sheet structure [
97]. Choi et al. used 1 wt% bio-based cationic starch (CS) and anionic MMT, forming 5, 10 and 20 BLs. The LbL-coated cotton samples reduced afterglow time in vertical flame tests (VFTs) but burned completely [
93]. In the majority of studies dedicated to cotton flame retardancy by means of LbL, BPEI is used either as a primer layer for better adhesion of chemical compounds to the cotton surface or as one of the oppositely charged pairs for a bilayer recipe.
Another chemical compound used either as a primer layer or as one of the oppositely charged polyelectrolyte is a positively charged coupling agent rich in nitrogen and silicon 3-aminopropyl triethoxysilane (APTES), which is mostly used as a sol–gel precursor in the preparation of sol–gel materials and coatings [
98]. Li et al. coated cotton fabric with 5, 10 and 15 BLs of 5 wt% cationic APTES and 2 wt% anionic phytic acid (PA), with drying at 100 °C after each dipping step. The 15 BL treatment was able to stop fire immediately after removing the ignition source [
99]. PA is an inexpensive and easily obtained phosphorus-rich chelating agent from plant/seed sources, with high absorption of polycationic compounds [
100]. In 2019, the same group of authors used a 1 wt% cationic suspension of PEI with alumina-coated silica nanoparticles (SiO
2) at pH 5 (instead of APTES) and anionic 2 wt% PA at pH 6 forming two, four and seven BL. The minimum number of BLs passing VFTs was 2, with the limiting oxygen index (LOI) value of 26.0%. The study also showed a minor loss in breaking strength in the warp and weft directions (~15%) relative to untreated cotton, likely caused by the formation of hydrogen bonds between SiO
2–PEI/PA and cotton fibers and the breaking of intermolecular and intramolecular hydrogen bonds in cellulose. The minor reduction in the loss of breaking strength after LbL treatment is an advantage over the commercial processes, which exhibit higher loss in mechanical strength [
94]. The same authors used 4 wt% anionic polyphosphoric acid (PPA) instead of PA, thus reducing the number of BLs from two to one with the same FR and mechanical performance [
101]. Anionic PA, as a green alternative to commercial phosphorus-based FR for cotton, has been used by several authors. Liu et al. coated cotton with a cationic solution of 0.5 wt% PEI, with added low-molecular-weight 2.0 wt% melamine (ME) at pH 4 in combination with 3.0 wt% PA at pH 4, forming two and four BLs. Only the samples coated with four BLs passed VFTs [
90]. In another study 5, 10 and 15 QLs of cationic 5.0 wt% APTES, 2 wt% anionic PA, 1 wt% cationic chitosan (CH) and again anionic PA (the pH of all solutions was 3.5) were deposited with subsequent dipping and drying at 100 °C. The LOI value fabric coated with 15 BLs was 29.0% and it passed VFTs [
86]. CH is another positively charged polymer from renewable sources (shells of shrimp and other sea crustaceans) widely used for LbL deposition due to its pKa value of 6.5 from amino group in a linear polysaccharide molecule composed of β-(1-4)-linked d-glucosamine (deacetylated unit) and N-acetyl-d-glucosamine (acetylated unit) [
102]. Chen et al. pre-treated cotton by immersing it into positively charged APTES to improve the adhesion of two, three and six BLs consisting of polyanionic ammonium polyphosphate (APP, 1 wt%) and polycationic CH (0.5 wt%). Each immersion step was followed by drying at 100 °C. Only three and six BLs passed VFTs [
87]. Anionic PA (2 wt%), with added sulfonated melamine-formaldehyde (SMF, 1 wt%), was used in the study of Pan and Zhao in 2018. Cotton fabric was coated with 5 and 10 BLs of cationic CH (0.5 wt%, pH 5) and anionic PA–SMF (pH 5). Only fabric treated with 10 BLs passed VFTs [
91]. Zhang et al. successfully coated cotton with eight BLs of positively charged PEI (0.5 wt%, pH 9) and negatively charged PA (2.0 wt%, pH 4) by dipping in deionized water (DI) and drying after each dip. The coated fabrics passed VFTs [
88]. Zilke et al. pre-treated cotton fabric with BPEI (1 wt%) to add a positive charge on the fabric surface and then dipped it into anionic PA (5 wt%, pH 0.7) and cationic 5 wt% polyvinyl amine (PVAm), forming 5, 10 and 15 BLs. The lowest number of BLs passing horizontal flame spreading test was 10 [
89]. PVAm is a linear polymer with the highest content of primary amine functional groups of any polymers [
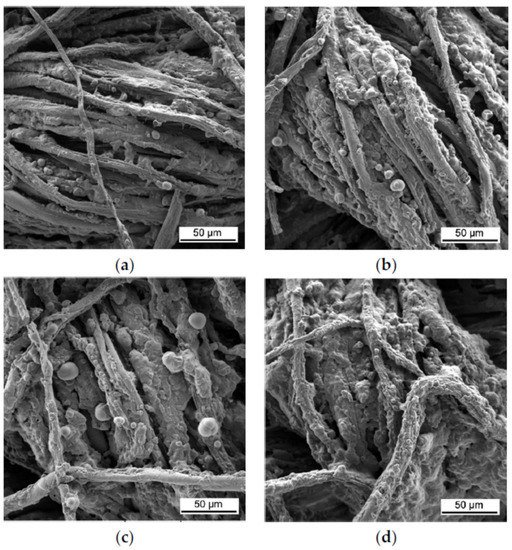
103]. Magovac et al. used 8, 10 and 12 BLs of anionic PA (2 wt%, pH 4) and cationic CH (0.5 wt%) with added urea (U, 10 wt%), where 10 BLs passed VFTs and the char left after performing the test showed characteristic intumescent bubbling, as shown in
Figure 2 [
92].
Figure 2. SEM images of the char of cotton samples treated with (
a) 8, (
b) 10, (
c) 12 and (
d) 15 BLs of PA/CH-U after performing vertical flame testing [
92].
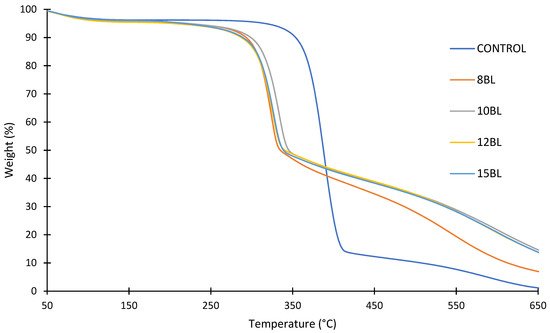
The thermogravimetric analysis (TGA) showed an increase in char with the increase in bilayers at 650 °C (from 1.2% for untreated cotton to 7.0% for 8 BLs and 14.1% for 10, 12 and 15 BLs), as well as the reduction in degradation temperatures, as shown in
Figure 3 [
92]. CH-U/PA decreased the decomposition rate of cotton by generating more non-flammable gases (e.g., CO, CO
2, NO
x) instead of highly flammable levoglucosane, which diluted the concentration of the combustible gases and absorbs heat, causing bubbling. At the same time, urea catalyzed the reaction of PA, as well as the decomposition of cellulose at lower temperature, thus forming intumescent char, which acted as a physical barrier that blocked heat and oxygen [
104].
Figure 3. TGA of untreated and treated cotton fabrics with 8, 10, 12 and 15 BLs of PA/CH-U [
92].
In 2020, Liu et al. studied the reduction in the flammability of cotton by using fully environmentally benign compounds—egg white protein and PA. Egg white protein is rich in amino acids, phosphorus, sulfur and metal complexes, such as calcium, iron, etc. Due to its complex nature, the protein attracts negatively charged PA. The study showed that even one BL of egg white protein and PA (70 wt%) gave excellent FR performance. TGA in air demonstrated a char yield of almost 33% at 600 °C and microscale combustion calorimeter (MCC) data revealed a 23% reduction in pHRR and a 67% reduction in THR [
105].
Another high charge density cationic nitrogen-rich polymer used in LbL polyelectrolyte pairs is polydiallyldimethylammonium chloride (PDAC), widely used for coagulating and removing negatively charged particles and dissolved organic matter from drinking water [
106]. Carosio et al. used a 1 wt% cationic solution of PDAC, 1 wt% anionic poly (acrylic acid) (PAA) and anionic 1 wt% suspension of APP. Cotton treated with a combination of PDAC/PAA/PDAC/APP in 1, 5 and 10 quadlayers (QLs), passed the horizontal flame test (HFT) [
107]. In their second study, instead of APP, they used nitrogen and phosphorus-rich anionic 1 wt% DNA, forming 5 and 10 BLs. Such treated cotton was then immersed into 0.1 wt% and 1 wt% hydrotalcite (HT) nanoparticle suspensions. HT is a zeolite that is used as an antacid in medicine. The 0.1 wt% HT concentration lowered the minimum number of BLs required for obtaining cotton self-extinguishment. All samples passed the HFT, but the 0.1 wt% HT concentration showed the best performance in terms of pHRR (33%) and THR reductions (27%), relative to untreated cotton [
108]. Jang et al. used 0.25 wt% cationic polyvinyl alcohol (PVA) and a 0.1 wt% anionic suspension of graphene nanoplatelets (GNP) and poly (4-styrenesulfonic acid) (PSS) to build 10 BLs on cotton. It resulted in a reduction in pHRR by 34.4% and in THR by 47.4%, respectively, but did not pass VFTs [
95].
Another interesting study made by Pan et al. was to investigate the effect of barium, nickel and cobalt ions crosslinked with alginate on the thermal stability and flammability of cotton fabric. Cotton was coated with PEI (0.5 wt%, pH 9) and anionic sodium alginate (SA; 0.3 wt%, pH 7), forming 10 BLs. The samples were then immersed into 5 mol/L solutions of metal salts, such as barium chloride (BaCl
2), cobalt acetate tetrahydrate (C
4H
6O
4Co x 4H
2O), or nickel acetate tetrahydrate (C
4H
6O
4Ni x 4H
2O), for crosslinking. Adding metal ions led to improved char residue and reduced DTG peak compared to untreated cotton, as well as the reduction in HFT burning rate. In terms of durability of treatment, the metal crosslinked LbL coating was durable even up to 6 h of washing in a detergent solution [
109]. Alginate is a linear polysaccharide consisting of a-l-guluronic acid and β-d-mannuronic acid residues produced by brown algae and bacteria. It is used in the food industry as a thickening agent, gelling agent, emulsifier, stabilizer and texture-improver, as well as in medicine for wound dressing [
110]. Pan et al. continued their studies on reducing the flammability of cotton and, instead of anionic alginate, they used a 2 wt% anionic hypophosphorous acid-modified chitosan (HACH) solution, forming 5 and 10 BLs of PEI/HACH. The cotton fabric was then immersed into a 1 wt% solution of genipin, a natural cross-linker for chitosan, proteins, collagen, etc. Ten bilayer coatings extinguished the fire of cotton during the horizontal burning test. PHRR and THR of 10 BL fabrics were reduced by 73% and 80%, comparing with those of pure cotton. Cotton coated with 10 BLs and cross-linked with genipin exhibited FR properties up to two washing cycles [
111].
Wang et al. coated cotton fabrics with a 1 wt% cationic solution of CH with added p-aminobenzene sulfonic acid-modified melamine (AMM, 3.3 wt%) and 3.3 wt% anionic APP to build 5, 10 and 15 BLs. The resulting 15 BLs on cotton fabric exhibited excellent FR properties (LOI 31.5%, 40% decrease in pHRR, 60% THR reduction with 24.1 wt% char residue and passed VFTs), as well as showing low cytotoxicity in a cell culture [
112]. In 2020, Lazar et al. pre-treated cotton fabric with a 1 wt% solution of PEI to generate a positive charge on a cotton surface and then coated it with 5, 10 and 15 BLs of 2 wt% anionic PSP (pH 4) and 0.1 wt% cationic CH (pH 4). Each dipping step was followed by rinsing in deionized water. The resulting FR cotton fabric passed VFTs. By adding a 100 mM solution of tris(hydroxymethyl)aminomethane (THAM, pH 4) to the rinsing steps, the number of bilayers needed to achieve the same FR properties of treated cotton fabrics passing VFTs was reduced to 10 [
113].
Most of the studies dedicated to cotton flame retardancy by means of LbL deposition show that compounds consisting of nitrogen, phosphorus, sulfur or inorganic particles reduced the pHRR values from 23% to 73% and the THR values from 27% to 80%, relative to untreated cotton. There is no general rule regarding how to obtain an optimal combination of FR ingredients to achieve a self-extinguishing behavior of cotton comparable to commercial flame-retardant finishing. The FR properties of LbL-treated cotton depended on the ionic strength of charged layers, number of layers, chemicals used, concentration of polyelectrolytes in solution, pH of solution, dipping time, pre-treatment of cotton fabrics and post-treatment of treated fabrics. By increasing the number of layers, as well as the concentration of chemical compounds in charged solutions (up to 10 wt%), the thermal stability of cotton increased. In terms of durability of LbL treatments, only a few studies show that the durability of FR on cotton was poor and the chemicals were washed away. The durability of FR treatment up to two laundry cycles in water or detergent solution was improved by post-curing with a crosslinking agent [
111].
3. Multifunctional Finishing of Cotton
Besides excellent flame retardancy, cotton fabrics should very often satisfy many other properties, such as antistatic, antibacterial properties, hydrophobicity, self-cleaning and antifouling properties, wrinkle resistance, UV protection, electromagnetic interference shielding and conductivity [
114]. The multifunctionality of cotton is possible to achieve via LbL deposition of commercially available compounds or simply by immersion of LbL-treated cotton into an active substance as a post-treatment. The wash durability of such multifunctional properties could be much better with crosslinking performed during thermal curing. An et al. coated cotton first with PEI (0.01 M) as a primer and then with 5 mg/mL of a graphene oxide (GO) anionic suspension and 3 wt% cationic caprolactam modified casein emulsion (CA), forming 1, 5 and 10 BLs. The final step was immersion into a sodium borohydride (NaBH
4) solution, followed by APP (7 wt%) solution. The obtained LOI of this treated cotton fabric (10 BLs) was 23.6%, passing VFTs, whereas pHRR was reduced by 64% and THR was reduced by 38%, relative to untreated cotton. These fabrics also obtained excellent antistatic properties [
115]. Zang et al. coated cotton fabrics with 10 QLs (two-sided) of cationic APTES (5 wt%, pH 4), anionic SA (3 mg/mL, pH 7), anionic APP (1 wt%, pH 9) and different concentrations of anionic GO (0.5; 1; 1.5 mg/mL) by spraying, which resulted in a fabric with improved FR, antistatic and antibacterial properties. Among all the treated fabrics, cotton treated with a 1 mg/mL concentration of anionic GO had the best self-extinguishing performance, with a pHRR value of about 3.2% of uncoated cotton (the reduction in pHRR was ~96.8% and that in THR was ~93%) [
116].
Another interesting study dealing with FR and the antimicrobial properties of LbL-coated cotton comes from Li et al. Cotton fabrics were first pre-treated with 1 wt% cationic PEI as a primer and then coated with 10, 20 and 30 BLs of an anionic PA solution (2 wt%, pH 4) and a 1 wt% cationic poly[3-(5,5-cyanuricacidpropyl)-siloxane-co-trimethylammoniumpropylsiloxane chloride (PCQS) solution. At 30 BLs, the break strength of the treated cotton decreased by 14% in the warp and by 6% in the weft direction. The fabric that passed VFTs (30 BLs), with an LOI value of 29.8%, was then immersed into a 0.5 wt% antibacterial sodium hypochlorite solution (NaClO) at pH 7. The strength of cotton slightly decreased after chlorination [
117]. In 2020, the same researchers treated cotton with one BL of a cationic CH solution and a 3 wt% anionic ammonium phytate (AP) solution (pH 7) to reduce the number of BLs, thus obtaining fabric with efficient FR as well as antimicrobial properties. The LOI value of this treated cotton was 27%, with a char length of 78 mm after performing VFTs [
118]. In 2021, Magovac et al. used a 2 wt% anionic PA solution (pH 4) and 0.5 wt% cationic CH solution (pH 4) with added U (10 wt%) to form 8, 10 and 12 BLs. At the end of the layering, the FR-treated cotton fabric was immersed into a 2% CuSO
4 solution to achieve additional antibacterial property. The results showed that cotton treated with 12 BLs self-extinguished the flame in VFTs (char length, 6.5 cm), with an LOI value of 26% and a reduction in pHRR by 62% and in THR by 54%. The resulting cotton killed almost 100% of bacteria [
104].
Li et al. coated cotton fabric with cationic PEI with added SiO
2 and anionic PPA (1 BL), as mentioned in
Section 4.1. This fabric was finally treated with a commercially available water-repellent finish (6 wt%, pH 6) by a dip–pad–dry process. The resulting fabrics passed VFTs and exhibited excellent hydrophobic properties [
101]. Excellent FR, as well as hydrophobic properties, could be achieved by coating cotton with four BLs of cationic PEI with added melamine (ME) and anionic PA, then immersing into chloroform solution and curing [
90].
Another interesting study was conducted by Lin et al. by coating cotton fabric with only one BL of cationic BPEI (2 mg/mL) and anionic APP (80 mg/mL) and immersing it into polydimethyl siloxane (PDMS) with fluorinated silica (F-SiO
2), followed by crosslinking in an oven at 130 °C for 30 min. This fabric exhibited excellent FR (the sample passed VFTs; pHRR and THR values were reduced by 86% and 39%), hydrophobic, self-cleaning, self-healing and antifouling properties with acid/alkali resistance [
119]. The same properties could be achieved by coating cotton fabric with 16 BLs of cationic poly (dimethyldiallyl) ammonium chloride (PDDA) and anionic boron nitride nanosheets (BNNS) [
120]. To obtain FR, wrinkle-resistant, antibacterial and UV-protective properties of cotton fabric, Safi et al. dipped fabrics first into a cationic solution consisting of 1 wt% CH, 5 wt% citric acid and 2 wt% sodium hypophosphite (SHP), followed by drying at 110 °C; then, they dipped them into a solution of 5 wt% sodium lignin sulphonate (SLS) and 4 wt% boric acid (BA), followed by drying at 80 °C for each dip. At the end, the fabrics were cured at 150 °C for 4 min. Three bilayers passed VFTs with an LOI of 30.5, while the pHRR was reduced by 50% relative to untreated cotton [
121].
In a recent study by Xue et al., cotton fabric was coated with 10 BLs of cationic BPEI and anionic carbon nanotubes (CNT). Ten bilayers were then immersed into BPEI and APP, forming one BL. Each dipping was followed by drying at 60 °C. The resulting multilayered fabrics exhibited excellent FR (char length after combustion in VFTs was 7 cm) as well as conductive properties. By immersing the same fabric into one trilayer (TL) consisting of BPEI/APP/PDMS instead of BPEI/APP and subsequent drying and curing at 60 °C, the resulting fabric became superhydrophobic, acid/alkali/organic solvent-resistant, UV-protective and wash-resistant after long-time laundering [
122]. To obtain excellent FR properties, conductivity and electromagnetic interference shielding durable under continuous external forces or washing tests, Zhang et al. treated fabrics with eight BLs of PEI and PA followed by drying after each dip (already mentioned in
Section 4.1). The coated fabric was then immersed into a 0.8 wt% ethanol suspension of silver nanowires (AgNWs) from one to four times, followed by drying at 50 °C after each immersion step. Only four-time-immersed treated cotton suppressed flames, passing VFTs with an LOI value of 37% and a reduction in pHRR and THR by 41.4% and 27.1%, respectively [
88].
Studies dedicated to LbL deposition for multifunctional finishing of cotton show an unlimited choice of chemicals at a very low concentration (up to 10 wt%) applied either as layers or by immersing treated cotton into a charged solution at the end of the deposition. This multifunctional finishing reduced the pHRR values by 41–97% and the THR values by 27–93%. By combining different chemicals and varying their concentrations as well as number of layers, it was possible to achieve multifunctional cotton with LOI values greater than 29% [
117,
121]. A few studies show that LbL deposition had minimal influence on the break strength of treated cotton (at a certain number of layers, the break strength decreased by 14% in the warp and by 6% in the weft direction) [
117]. The major problem of LbL multifunctional finishing is wash durability, which could be improved by post-curing with an appropriate crosslinking agent [
88,
119,
122].
4. Layer-by-Layer Deposition to Reduce Flammability of Polyester
There are relatively few studies regarding the use of layer-by-layer deposition to reduce the flammability of polyester [
107,
123,
124,
125,
126,
127,
128,
129,
130,
131]. The compounds used for the treatment of polyester are long-chain organic water-soluble polymers (polyelectrolytes), short-chain organic molecules and suspensions of inorganic nanoparticles. For a better adhesion of these compounds to polyester, or as one of the oppositely charged pair of a BL, long-chain organic water-soluble charged polymers, such as PDAC, PAH, CH and PEI/BPEI, have been used [
107,
123,
125,
126,
127,
128]. Another option to increase the charge of polyester fabric is surface functionalization, such as alkali hydrolysis and UV-grafting [
130].
Carosio et al. used a 1 wt% positively charged PDAC solution, widely utilized for building LbL assemblies in combination with 1 wt% negatively charged PAA, capable of crosslinking at temperatures above 200 °C, as well as a 1 wt% negatively charged APP suspension. Polyester fabrics treated with a combination of PDAC/PAA/PDAC/APP in 1, 5 and 10 quadlayers (QLs), limited the flammability of the fabrics by suppressing the afterglow and melt dripping, as well as lowering heat release during combustion [
107]. In another study, three different 0.2 wt% cationic suspensions compounds—PDAC, SiO
2 and polyhedral oligomeric silsesquioxane cage molecules carrying eight n-propylammonium chloride groups (POSS
®)—with a 0.2% anionic suspension of α-zirconium phosphate (ZrP) were deposited as 5 and 10 BLs. The treated fabric showed an overall improvement in thermal stability by increasing the time to ignition (up to 86% for PDAC) and decreasing the pHRR (up to 26% for POSS). Alumina-coated silica nanoparticles reduced the production of smoke (up to 25%), but no VFT was performed [
123]. The same group of authors studied the influence of LbL spraying vs. dipping on the flammability of fabric. Polyester was coated with five BLs of a cationic 0.2 wt% suspension of alumina-coated silica colloidal nanoparticles and a 0.2 wt% suspension of anionic silica colloidal nanoparticles. This study demonstrated that building layers by spraying is more efficient for achieving a homogeneous coverage, as well as suppressing the dripping of the polyester fabric [
124].
Apaydin et al. combined cationic PAH, anionic sodium polyphosphate PSP, a flame retardant and a negatively charged suspension of titanium dioxide (TiO
2) in 5, 10 and 15 QLs of PAH/PSP/PAH/TiO
2. However, even at 15 QLs, the pHRR decreased only by 14%, which means that the treatment had little influence on flammability [
125]. PAH has been used as a cationic polyelectrolyte rich in nitrogen for the preparation of hollow microcapsules for biomedical drug delivery [
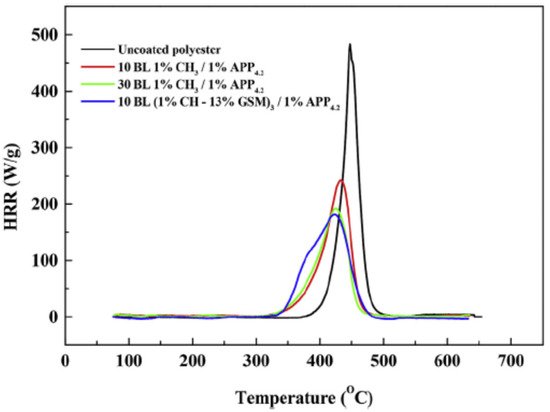
132]. Jordanov et al. showed that, by adding low-molecular-weight FR compounds into a 1 wt% cationic CH network, in combination with a 1 wt% anionic APP suspension, it was possible to reduce the number of bilayers needed to pass the VFT (from 30 BLs to 10 BLs). As low-molecular-weight FR additives, nitrogen and nitrogen/sulfur-based derivatives such as 13 wt% guanidine sulfamate (GSM), 13 wt% U, or 13 wt% thiourea (THU) were used in the cationic CH solution. The sample coated with 10 BLs of CH–GSM/APP showed the same self-extinguishing properties as polyester coated with 30 BLs of CH/APP. Moreover, the 10 BLs CH–GSM/APP coating reduced the pHRR by 61.7% relative to uncoated polyester, as shown in
Figure 4 [
126].
Figure 4. MCC curves of heat release rates as a function of temperature for uncoated and coated polyester [
126] Reproduced with permission from Jordanov, I.; Magovac, E.; Fahami, A.; Lazar, S.; Kolibaba, T.; Smith, R.J.; Bischof, S.; Grunlan, J.C. Flame, Polym. Degrad. Stab.; published by Elsevier Ltd., 2019.
Other authors used nitrogen-rich high-molecular-weight cationic PEI or BPEI for coating polyester. Wattanatanom et al. studied the influence of polyelectrolyte concentrations, as well as the number of layers, on the flammability, break strength and stiffness of LbL treated polyester fabric, including wash resistance of FR coating. Another study used a 0.5 wt% cationic BPEI solution and a 5, 7 and 10 wt% anionic APP suspension to reduce flammability and anti-dripping properties. The fabric was first padded in BPEI solution, dried at 80 °C and then padded in APP solution and dried at 110 °C to deposit three, five and seven BLs. Increasing the number of bilayers (three, five and seven BLs) or the concentration of the solution (5, 7 and 10 wt%) improves flame retardancy and anti-dripping of polyester by decreasing after-flame time of coated fabric and self-extinguishing the flame [
127]. In a second study with the same formulations, they showed that increasing the concentration of APP, as well as the number of layers, led to an increase in the break strength and stiffness of the fabric, indicating that FR finishing via LBL deposition did not degrade the strength. The formulation of 10 wt% APP at seven BLs showed wash durability of the FR coating for one washing cycle [
128]. Carosio et al. investigated how adding salt into solutions influenced the layers, improving the FR properties with the same number of BLs. The authors used 0.1 wt% cationic BPEI as a primer layer to functionalize polyester. The fabric was then immersed into a 0.7 wt% anionic MMT suspension, rinsed in deionized water and then immersed into cationic 1 wt% octapropylammonium polyhedral oligomeric silsesquioxane (OAPOSS) to deposit five BLs. Adding 0.10 M sodium chloride (NaCl) into both the cationic and anionic solutions modified the ionic strength of the systems, which resulted in thicker and more homogeneous coatings. A thicker coating decreased flame spread rate in horizontal flammability tests relative to fabric with the same number of BLs without added NaCl and the fabric showed no melt-dripping. The FR coating showed the same performance after a 1 h washing at 70 °C [
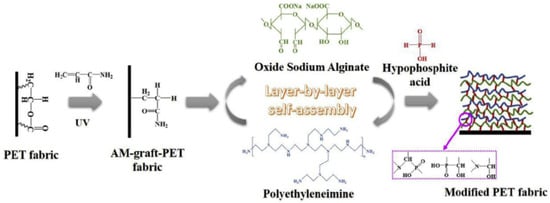
129]. Pan et al. alkali hydrolyzed polyester (PET) fabric, UV-grafted it with commercial thickening agent acrylamide (AM) and benzophenone and coated this pre-treated fabric with 5, 10 and 15 BLs of a 0.5 wt% cationic PEI solution and a 0.3 wt% anionic oxide sodium alginate (OSA) solution, a natural polysaccharide found in brown algae. After LbL treatment, the fabrics were immersed into 10 wt% hypophosphorus acid for crosslinking, as shown in
Figure 5. Fabric treated with 15 BLs did not show any melt-dripping in horizontal flammability tests and the fire self-extinguished. The pHRR and THR values decreased by 44% and 29.4% relative to untreated fabric and the FR treatment was durable for 12 laundering cycles [
130].
Figure 5. Schematic of LbL deposition of pre-treated MA-graft-polyester (PET) fabric coated with 15 BLs of OSA/PEI and post-treated with HA cross-linking [
130] Reproduced with permission from Pan, Y.; Liu, L.; Song, L.; Hu, Y.; Wang, W.; Zhao, H., Polym. Degrad. Stab.; published by Elsevier Ltd., 2019.
The influence of dipping time in polyelectrolyte solution on the flammability of polyester fabric was investigated by Jiang et al. One trilayer (TL) was built by immersing the fabric into a 5 wt% cationic sol solution of flexible polysiloxane (SSP) prepared by sol–gel from methyltriethoxysilane (MTES), isopropanol (IPA) and hydroxy-terminated polydimethylsiloxane (PDMSOH) and a 10 wt% anionic PA solution. One TL consisted of SSP/PA/SSP. The dipping time was set to 0, 5, 10, 15 and 20 min. The study showed that the FR properties of the fabric improved with soaking time, so 20 min of soaking exhibited self-extinguishing properties of polyester fabrics during VFTs, with a 65% reduction in pHRR in comparison with untreated polyester. The FR effect of this fabric was durable up to 45 washing cycles [
131].
Studies dedicated to polyester flame retardancy by means of LbL deposition show that compounds consisting of nitrogen, phosphorus, sulfur, or inorganic particles reduced the pHRR values by 65% and the THR values by 29%, relative to untreated polyester. By combining different compounds, varying their concentration and applying varying number of bilayers, polyester fabric exhibited no melt dripping and a self-extinguishing behavior. The same effect could be achieved by increasing the immersion time of the fabric in a polyelectrolyte solution [
131]. Generally, LbL deposition did not degrade the strength of the polyester fabric [
128]. Studies showed that the wash durability of FR treatment depended almost exclusively on the creation of covalent bonds between layers, which could be improved by post-curing with adequate crosslinking agent [
131].
5. Layer-by-Layer Deposition to Reduce Flammability of Polyamide Textiles
The compounds used for FR LbL deposition of polyamide are similar to those applied to cotton and polyester. According to the literature, polyamide is mainly treated with cationic polymers, such as PAH, CH and PEI, as a primer layer or one of the polyelectrolyte pairs [
125,
133,
134,
135,
136]. As a pre-treatment, chemical grafting with PAA as well as enzymatic modification have been reported [
137,
138]. Apaydin et al. experimented with a 1 mg/mL cationic PAH solution and a 1 wt% anionic MMT suspension to deposit 5, 10 and 20 BLs on PA6. Cone calorimetry revealed that 20 BLs reduced the pHRR values by more than 60% [
133]. These same researchers deposited cationic PAH with anionic PSP to build 5, 10, 15 and 40 BLs on PA6.6. TGA showed that the amount of residue increased for 20 and 40 BLs, while the cone calorimeter data showed a significant decrease in pHRR (up to 36%) for all coated fabrics [
134]. The same group of authors combined cationic PAH, anionic PSP and an anionic suspension of titanium dioxide (TiO
2) to deposit 5, 10 and 15 QLs of PAH/PSP/PAH/TiO
2 (
Section 4.3). Cone calorimetry showed that the coating reduced pHRR by 26% for PA6.6 fabric treated with 15 QLs, but the presence of TiO
2 did not significantly improve the FR performance relative to the formulation without TiO
2 [
125]. Kumar Kundu et al. deposited 5, 10 and 15 QLs of cationic CH, anionic PA and anionic oxide sodium alginate (OSA) on PA6.6. The aldehyde groups in OSA formed strong covalent bonds with CH and it could be used in LbL deposition as a cross linker. In the VFT, 10 and 15 QL coatings stopped the melt-dripping of PA6.6, with LOI values of ~22%. Cone calorimetry showed that a maximum reduction (24%) in the pHRR was achieved with five QL deposition [
135]. In 2018, the same group of authors treated PA6.6 with 5 and 10 BLs of a 1 wt% cationic CH solution and a 2 wt% anionic PA solution to build 5 and 10 BLs. The fabrics were further impregnated in 1 and 5 wt% Na-tetraborate decahydrate solutions and cured at 90 °C. All the treated fabric samples could stop melt dripping in VFTs and pHRR values were lowered compared with the control. In terms of FR performance, the best results were with fabrics treated with 10 BLs (a 31% reduction in pHRR relative to untreated fabric). This coating remained durable up to five washing cycles for PA6.6 impregnated with borate [
136]. In 2020, they deposited a 1 wt% cationic CH solution and an anionic solution of 1 wt% phosphorylated chitosan (PCH) and 0.25 wt% poly-acrylate sodium (PAS) onto PA6.6 via “one pot” and LbL deposition to compare the efficiency of these two methods in the reduction in the flammability of PA6.6. Fabric treated via “one pot” for 5 and 10 min was then UV-grafted. Layered fabric was immersed first into cationic CH, washed with DI and then immersed into anionic (PCH-PAS), forming 5 and 10 BLs, then either UV-cured (5 and 10 BLs) or thermally crosslinked (10 BLs). The results indicated that the UV-grafted fabric treated with 10 BLs, with a higher weight gain%, exhibited the highest LOI value of 23% and a 25% reduction in pHRR relative to untreated fabric. However, only the thermally cross-linked PA6.6 treated with 10 BLs retained the FR performance after 5 washing cycles [
139]. One-pot synthesis is an expression denoting that all the reactants are subjected to successive chemical reactions in just one reactor, thus saving time and resources and improving the efficiency of a chemical reaction [
140].
Ziaur Rahman et al. investigated the influence of pre-treatment and post-treatment on the thermal properties of PA6.6 deposited with two and five BLs of a cationic CH solution with added ME and U and an anionic PA solution. The fabrics were first chemically grafted with PAA in a solution of benzene and dibenzoyl peroxide (BPO). The fabrics were then dipped into cationic PEI, dried at 70 °C and then dipped into a polyacrylic acid–co-maleic acid solution (PAACM) and dried. After LbL treatment, the fabrics were impregnated in a cationic CH and graphene oxide (GO) solution through a pad–dry–cure process. Despite excellent hydrophilic properties achieved by adding GO, none of the treated fabrics passed VFTs either before or after washing [
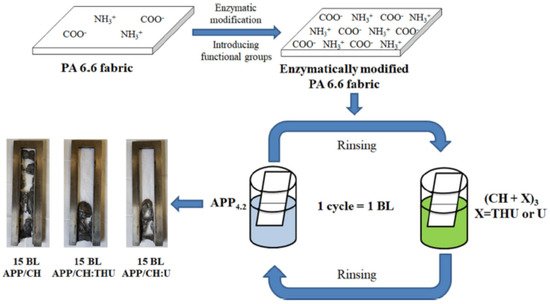
137]. In 2020, Jordanov et al. successfully deposited 15–25 BLs of a 1 wt% anionic APP suspension and a 1 wt% cationic CH solution, with added low-molecular-weight compounds 20 wt% THU or U, onto the enzymatically modified surface of PA6.6 fabrics. The process is schematically shown in
Figure 7. By adding low-molecular-weight FR compounds into the CH network, the number of BLs passing the HFT was reduced from 25 BLs of APP/CH-U to 15 BLs, while the pHRR was reduced by 35% relative to untreated fabric [
138]. A summation of polyelectrolytes used to achieve FR of polyamide fabrics is provided in
Table 4.
Figure 7. LbL deposition scheme of APP/CH+X on enzymatically modified PA6.6 fabric [
138] Reproduced with permission from Jordanov, I.; Kolibaba, T.J.; Lazar, S.; Magovac, E.; Bischof, S.; Grunlan, J.C., J. Mater. Sci.; published by Springer Nature Switzerland AG., 2020.
Table 4. Polyelectrolytes used to achieve FR of polyamide fabric.
There are relatively few studies about LbL deposition of FR compounds on polyamide fabrics, showing reductions in pHRR from 24 to 60% relative to untreated fabric. The results of VFTs and HFTs showed decreased melt dripping. By varying different coating parameters (FR compounds, concentration and number of bilayers), polyamide fabrics could be self-extinguishing. Wash durability (up to five washing cycles) of FR LbL treatment could be achieved by low-temperature thermal curing [
139].
6. Layer-by-Layer Deposition to Reduce Flammability of Cotton/Polyester and Cotton/Polyamide Blends
There are very few studies dealing with LbL deposition of cotton/polyester blends to obtain FR properties. Carosio et al. treated cotton/polyester fabrics with a quadlayer (QL) combination of PDAC/PAA/PDAC/APP. The resulting coating limited the flammability of the fabric by suppressing the afterglow and melt dripping, as well as lowering heat release during combustion (
Section 4.1) [
107]. Wattanatanom et al. studied the influence of polyelectrolyte concentration, as well as the number of layers, on the flammability of cotton/polyester blends. By using a cationic BPEI solution and a 5, 7 and 10 wt% anionic APP suspension, the flammability and anti-dripping properties of the fabric were reduced with three, five and seven BLs (
Section 4.3). The study showed that the increase in the number of bilayers or the concentration of the solution improved the flame retardancy and anti-dripping of blends by decreasing the after-flame time of coated fabrics and self-extinguishing the flame [
127]. Alongi et al. investigated whether different orders of layers with the same compounds and same concentration had any influence on the reduction in flammability and anti-dripping behavior of cotton/polyester blends. They used a 0.2 wt% cationic suspension of alumina-coated silica nanoparticles, 0.2 wt% cationic CH solution, 0.2 wt% anionic suspension of silica nanoparticles and 0.2 wt% anionic APP suspension to deposit 5 and 10 silica+/silica-/CH/APP QLs and 5 + 5 and 10 + 10 (CH/APP + silica
+/silica
−) BLs on fabric blends. The coated fabric did not pass VFTs, proving that only the thickness of the coating and weight gain had an influence on FR properties [
141]. In 2012, Carosio et al. coated two blend fabrics, one with a 0.2 wt% cationic CH solution and a 0.2 wt% anionic APP suspension, depositing 5, 10 and 20 BLs, and a second fabric with a 0.2 wt% cationic suspension of alumina-coated silica nanoparticles with 0.2 wt% APP. Despite the fact that both FR coatings suppressed the afterglow phenomenon, leaving a remarkable residue after combustion, none of the fabrics passed VFTs [
142]. In a previous study by Carosio et al. already mentioned in
Section 4.1, the burning rate of cotton/polyester blends was successfully reduced in HFTs relative to untreated fabric by combining PDAC/PAA/PDAC/APP in 1, 5 and 10 QLs [
107].
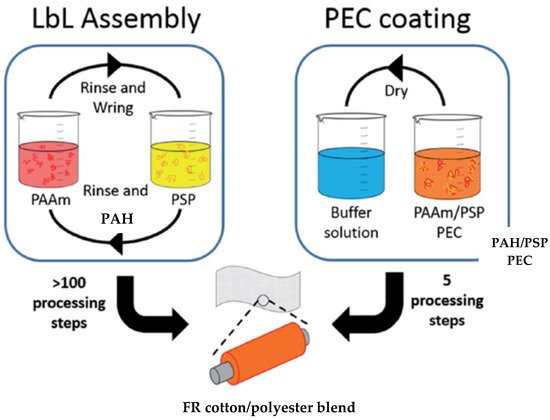
In 2016, Haile et al. compared the efficiency of two types of coating, LbL and “one pot” deposition, in extinguishing flames during VFTs, as well as the wash durability to home laundering of FR finishes. Blend fabrics were coated by means of LbL deposition with a 1 wt% cationic PAH solution and a 2 wt% anionic PSP suspension (20, 25 and 30 BLs) and by a “one pot” deposition of a water-soluble polyelectrolyte complex suspension (PEC) consisting of three different wt% concentrations (low, medium and high) of PAH and PSP. The LbL-coated fabric was dried at 70 °C, while “one pot” fabrics were dried and then immersed into a buffer solution consisting of citric acid and sodium citrate at pH 4 for 5 min, as shown in
Figure 8. In the acidic environment, PAH and PSP formed an insoluble complex, durable up to five laundry cycles. VFTs showed that the highly concentrated “one pot”-coated cotton/polyester fabric with 17.9% weight gain was able self-extinguish, while the MCC data showed a reduction in pHRR of 78% and 31% for cotton and polyester, respectively. The coating process was reduced from more than 100 processing steps to only 5 [
143].
Figure 8. LbL and “one pot” deposition scheme of cotton/polyester fabric with PAH and PSP [
143] Reproduced with permission from Haile, M.; Leistner, M.; Sarwar, O.; Toler, C.M.; Henderson, R.; Grunlan, J.C., RSC Adv.; published by Royal Society of Chemistry, 2016.
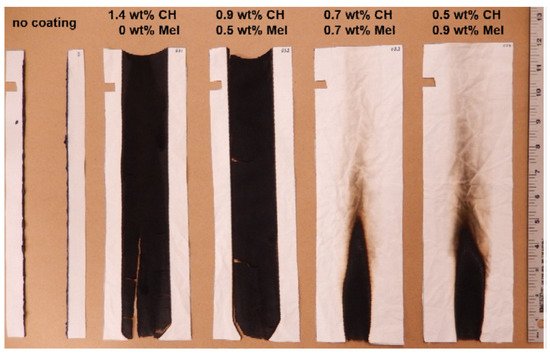
Leistner et al. investigated the influence of low-molecular-weight additives (e.g., melamine) into the cationic CH network for effective FR properties of cotton/polyester blends coated with a 1.4 w% cationic CH solution and a 2 wt% anionic PSP solution. In this study, the concentration of the cationic solution was held constant at 1.4 wt%, but the concentrations of single components in the cation solution (CH and ME) were different, as shown in
Figure 9. The number of bilayers required for a 12.5 wt% coating was 8 BLs for 1.4 wt% CH and 15 BLs for 0.5 wt% and 0.9 wt% melamine, where the latter showed the best result in VFTs, with a char length of 4.5 in and char residue of 93% after performing a combustion calorimeter test [
144].
Figure 9. Results of VFT of CH/PSP coating on fabric with melamine addition [
144] Reproduced with permission from Leistner, M.; Abu-Odeh, A.A.; Rohmer, S.C.; Grunlan, J.C., Carbohydr. Polym.; published by Elsevier Ltd., 2015.
Liu et al. also used ME as a low-molecular-weight additive in a cationic PAH solution. Cotton/polyester fabric was first pretreated with a 1 wt% anionic PAA solution for a better adhesion of LbL layers. The fabric was then immersed into a 1 wt% cationic PAH or PAH–ME solution and a 1 wt% anionic APP suspension, forming 10 BLs. Fabric treated with PAH–ME/APP self-extinguished, with a char length of 11.3 cm in VFTs and with an LOI value of 28.4%. The pHRR was reduced by 34.4%, with a 9 wt% coating [
145]. The same group of authors pre-treated cotton/polyester fabric with 0.1 wt% anionic PAA and then immersed it into a 0.5 wt% cationic BPEI solution and a 1 wt% or 2 wt% anionic hypophosphorous acid-modified chitosan (PCH) solution, depositing 10 and 20 BLs. During HFTs, the flame was completely extinguished for the sample coated with 20 BLs of 2 wt% PCH [
146]. By depositing alkali-hydrolyzed cotton/polyester blends with a 0.5 wt% cationic PEI solution and a 0.3 wt% anionic OSA solution, thus forming 5 and 10 BLs, and then soaking coated fabrics into a 10 wt% HA solution for cross-linking, it was possible to achieve self-extinguishing in HFTs with FR coating durable through 12 home laundry cycles [
147]. Wang et al. combined a 1 wt% cationic γ-paperazinylproplymethyldimethoxy silane (GP-108) solution with a 1 wt% anionic APP solution to build up 5, 10 and 15 BLs. Fabric coated with 15 BLs achieved self-extinguishing in VFTs and showed a strong decrease in heat release during cone calorimetry tests [
148].
The number of studies on LbL deposition to reduce the flammability of cotton/polyamide blends is very limited. Narkhede at al. first pre-treated these blends by immersing them into a pH 2 solution for cationization. The cationized fabric was then deposited with 5, 10, 15 and 20 BLs by dipping. For the anionic polyelectrolyte, a 2 wt% PSP solution was used and, for the polycationic, three different cationic polysiloxane compounds were used, namely, 6.8 wt% (trimethylammonium methyl phenythyl)-methyl siloxane and dimethyl siloxane copolymer chloride salt (QMS-435) solution, 4 wt% aminoethylaminopropyl silsesquioxane–methylsilsesquioxane copolymer oligomer (WSA-7021) solution and 4 wt% aminopropyl silesquioxane oligomers (WSA-9911) solution. Only fabrics coated with 20 BLs of WSA-7021 and WSA-9911 passed VFTs [
149]. A summation of polyelectrolytes used to achieve FR of blend fabrics is provided in
Table 5.
Table 5. Polyelectrolytes used to achieve FR of cotton/polyester and cotton/polyamide blends.
The reduced flammability of cotton blends can be easily achieved with a wide range of chemical compounds containing nitrogen, phosphorus, sulfur and inorganic compounds, as summarized in
Table 5. As a pre-treatment, various primer layer chemicals have been used, such as BPEI or PAA, or the cotton blends have been treated with acid/alkali hydrolysis to achieve more functional groups on the fiber surface. By means of FR LbL deposition, the pHRR values were reduced by 78% and 31% for the cotton and polyester. Wash durability of FR LbL treated blends could be achieved by low-temperature thermal curing (up to 12 washing cycles) [
147]. However, the role of each FR chemical compounds in LbL recipes and their mode of action on suppression of flames on cotton blends require further analyses, but the generally accepted opinion is that these compounds act as passive barriers and/or intumescent of known modes of actions.