Priming is an adaptive strategy that improves plant defenses against biotic and abiotic stresses. Stimuli from chemicals, abiotic cues, and pathogens can trigger the establishment of priming state. Priming with 5-aminolevulinic acid (ALA), a potential plant growth regulator, can enhance plant tolerance to the subsequent abiotic stresses, including salinity, drought, heat, cold, and UV-B. However, the molecular mechanisms underlying the remarkable effects of ALA priming on plant physiology remain to be elucidated.
1. Introduction
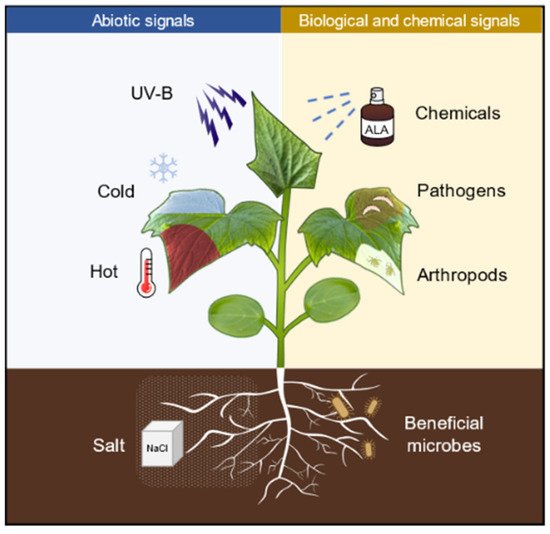
Defense priming refers to a physiological state (a state of readiness for defense) that is induced after the plants perceive a variety of stimuli, such as pathogens, arthropods, and abiotic cues, as well as chemicals (
Figure 1). In this state, the defense responses are deployed in a faster, stronger, and/or more sustained manner, thus defense priming is considered an adaptive and low-cost defensive strategy [
1]. The pathogen produces molecules with different biochemical natures (peptides, polysaccharides, or lipids) that are sensed by plants through the corresponding receptors, thus inducing plant priming [
2]. The common bean (
Phaseolus vulgaris) activates enhanced plant defense by inoculation with
Rhizobium etli and develops stronger resistance to
Pseudomonas syringae compared with unstimulated plants [
3]. Herbivore-inducible plant volatiles (HIPVs) released in response to herbivore attack can induce priming in neighboring plants, which exhibit faster or stronger defense activation and insect resistance when subjected to insect feeding [
4]. Repeated exposure of plants to mild abiotic signals, such as heat, cold, or salt, can also trigger plants into a defense priming state [
5]. In addition, pretreatment with low concentrations of chemicals such as hydrogen peroxide (H
2O
2), sodium hydrosulfide (NaHS), sodium chloride (NaCl), sodium nitroprusside (SNP), γ-aminobutyric acid (GABA), melatonin, polyamines (PAs), as well as 5-aminolevulinic acid (ALA) also induces plants into defense priming status [
6,
7,
8], in which plants respond to biological and abiotic stresses through faster and stronger defensive activation [
9]. Furthermore, the ability of priming to enhance stress tolerance can be self-propagating. For example, defense priming occurs in roots, while transcriptional differences can be detected in both roots and leaves [
10]; mobile wound signals transmitted from local damaged sites to distal undamaged sites induce the whole plant into priming [
11]; infestation of plants with phloem-feeding whitefly (
Bemisia tabaci) triggers local and systemic defense priming [
12]. Interestingly, the priming status of plants can be inherited across generations. For example, a transgenerational priming response against pathogen attack can last for at least two generations in common beans upon treatment with the priming agent GABA [
13]. This transgenerational inheritance of defense priming may involve epigenetic regulation [
2,
14].
Figure 1. Numerous biotic and abiotic stress as well as defense-related chemicals are capable of inducing plants into priming status. UV-B, ultraviolet B (UVB); ALA, 5-aminolevulinic acid; NaCl, sodium chloride.
2. Biosynthesis of 5-Aminolevulinic Acid
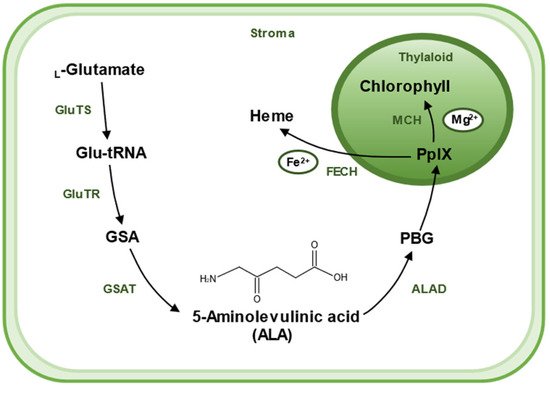
There are two pathways for the biosynthesis of ALA, the C4 pathway (or Shemin pathway) and the C5 pathway (or Beale pathway) [17]. The C4 pathway is found in animals, fungi, and some algae and bacteria. In this pathway, ALA is produced by direct condensation of succinyl-CoA and Gly catalyzed by ALA synthase. The C5 pathway is mainly found in plants and archaea, which consists of a three-step enzymatic reaction [18]. Firstly, L-glutamate is ligated to tRNAGlu, which is catalyzed by glutamyl–tRNA synthetase (GluTS) to form L-glutamy–tRNA. Secondly, the carboxyl group of Glu-tRNA is reduced to a formyl group and L-Glu-tRNA is converted to L-glutamic acid 1-semialdehyde (GSA). GluTR plays a key role during the synthesis pathway of ALA. Lastly, GSA undergoes an isomerization reaction catalyzed by glutamate-1-semialdehyde aminotransferase (GSAT) to form ALA. These reactions are located in the chloroplast stroma [19] (Figure 2).
Figure 2. A sketch shows the biosynthetic pathway of ALA and the use of ALA as a substrate for the synthesis of chlorophyll and heme in plants. ALA is created in stroma of chloroplast. The main biosynthetic pathway of ALA is the Beal pathway, which starts from glutamic acid. L-Glutamate is ligated to tRNAGlu, which is catalyzed by glutamyl–tRNA synthetase (GluTS) to form L-glutamy–tRNA. Then, Glu-tRNA is converted to L-glutamic acid 1-semialdehyde (GSA), a reaction catalyzed by the key rate-limiting enzyme glutamyl–tRNA reductase (GluTR). GSA then undergoes an isomerization reaction catalyzed by glutamate-1-semialdehyde aminotransferase (GSAT) to form ALA. Two molecules of ALA are catalyzed by ALA dehydratase (ALAD) and agglomerate to form a pyrrole ring called porphobilinogen (PBG). Then, after a six-step enzymatic reaction, four molecules of PBG polymerize to form a porphyrin structure, eventually forming (PpIX). PpIX combines with different enzymes and substrates to yield different products; PpIX chelates Fe2+ with Ferrochelatase (FECH) to produce heme, and Mg2+ with Mg-chelatase (MCH), and then undergoes a series of catalytic reactions to produce chlorophyll.
Chlorophyll and heme, with ALA as their precursors, are involved in many biochemical processes. They share a series of steps in the synthesis pathway from ALA to protoporphyrin IX (PpIX). It starts with two molecules of ALA catalyzed by ALA dehydratase (ALAD), which aggregates to form a pyrrole ring called porphobilinogen (PBG); this is followed by a six-step enzymatic reaction in which four molecules of PBG polymerize to form a porphyrin structure, eventually forming PpIX. The synthetic pathway branches off here to produce heme or chlorophyll, respectively. PpIX chelates Fe
2+ by Ferrochelatase (FECH) to produce heme or chelates Mg
2+ by Mg-chelatase (MCH) and undergoes a series of catalytic reactions to produce chlorophyll. ALA was originally obtained by chemical methods, which is a complex process and difficult to purify, resulting in a low yield and high price [
20]. At present, ALA can be produced commercially through easier, cheaper, and sustainable microbial methods [
21,
22,
23].
3. ALA Priming Enhances Plant Resistance to Abiotic Stresses
Abiotic stresses are considered to be major environmental factors limiting the yield and quality of crop plants. Developing effective strategies to mitigate the deleterious effects of abiotic stresses is critical for sustainable agriculture and food security. Recent studies have shown that exogenous treatment of plants with 5-ALA can enhance abiotic stress tolerance by inducing molecular and physiological defense mechanisms, providing a promising strategy for mitigating abiotic stress in plants.
3.1. ALA Priming Alleviates Salt Stress
Priming with ALA increases the transcripts and protein accumulations of SOS1 (Na
+/H
+ antiporter) and HA3 (proton pump) on the plasma membrane (PM) as well as NHX1 (Na
+/H
+ antiporter) and VHA-A (proton pump) on the vesicle membrane compared with the unprimed cucumber (
Cucumis sativus) in response to salt stress. The ion transporter proteins SOS1 and NHX1, with the energy provided by proton pump HA3 and VAH-A, help cucumber excrete Na
+ from the cytoplasm or transfer it to the vesicles, resulting in a high-low-high osmotic potential in the vesicle-protoplast-exosome, and thus alleviating ion toxicity induced by salt stress. Pretreatment with ALA upregulates the expression of high-affinity K
+ transporter protein 1 (HKT1) that regulates Na
+/K
+ homeostasis in cucumber cells and maintains normal metabolic activities in cells under salt stress conditions [
27,
28]. Proline accumulates in response to salinity and is a common compatible osmolyte in higher plants. Exogenous application of ALA upregulates delta-1-pyrroline-5-carboxylate synthase (P5CS) that controls the rate-limiting step of glutamate-derived proline biosynthesis in Oilseed rape (
Brassica napus) and enhances tolerance to salt stress [
26,
29]. In addition, priming with ALA relieves cell oxidation stress caused by salt stress by improving the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), and promoting the activity of enzymes involved in the ascorbate-glutathione cycle (AsA-GSH), including ascorbic acid oxidase (AAO), ascorbate peroxidase (APX), glutathione reductase (GR), dehydroascorbic acid reductase (DHAR), and monodehydroascorbic acid reductase (MDHAR) [
30,
31,
32,
33].
In addition to coping with osmotic stress and oxidative stress caused by salt stress, priming with ALA improves plant salt tolerance by increasing photosynthetic assimilation and promoting nitrogen metabolism. Cassia seed (
Cassia obtusifolia), peach (
Prunnus persica), and oilseed rape treated with ALA showed an increase in the net photosynthetic rate (Pn) and transpiration rate (Tr), as well as the photochemical efficiency of photosystem II (Fv/Fm) and the non-photochemical quenching (NPQ) during salt stress [
29,
31,
34].
The induction of salt tolerance in plants by ALA may be achieved through nitric oxide (NO). ALA treatment increased NO and NOS activity in leaves, suggesting that ALA triggers NO synthesis by activating NOS, and thus improves salt tolerance in maize (
Zea mays) [
38].
3.2. ALA Priming Increases Plant Tolerance to Extreme Temperature
ALA-pretreated cucumber leaves had higher antioxidant enzyme activity, higher levels of proline and soluble sugar content, and weaker growth inhibition under high-temperature stress conditions [
43]. Priming with ALA increases germination and seedling emergence in red pepper (
Capsicum annuum) and reduces tissue electrolyte leakage in rice (
Oryza sativa) under cold stress [
44,
45]. Pretreatment with ALA also increases chlorophyll content and photosynthetic capacity of cucumber and enhances ribulose-1,5-bisphosphate (RuBP) carboxylase activity in maize under cold stress conditions [
46,
47,
48]. Furthermore, ALA treatment also improved the antioxidant capacity of plants in response to cold stress by increasing the activities of SOD, APX, GR, CAT, and heme oxygenase-1 (HO-1) in red pepper, drooping wild ryegrass (
Elymus nutans), and soybean plants (
Glycine max) [
49,
50,
51]. Interestingly, ALA priming upregulates the expression levels of
respiratory burst oxidase homologue1 (RBOH1) in tomato (
Solanum lycopersicum) and leads to the production of H
2O
2, which serves as a signaling molecule to activate defense against cold stress [
52].
3.3. ALA Priming Mitigates Drought-Induced Damage
ALA pretreatment can maintain moisture in the seedlings of oilseed rape and Kentucky bluegrass (
Poa pratensis), thus enhancing leaf relative water content (RWC) [
58,
59]. It can also increase the contents of proline and foliar N in wheat (
Triticum aestivum), as well as Ca
2+ in the roots under drought conditions [
60,
61,
62]. In addition, in studies with Kentucky bluegrass and sunflower (
Helianthus annuus), priming with ALA increases the activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and glutathione reductase (GR), which reduce the production of ROS, including H
2O
2 content and O
2•− production, thereby improving tolerance against drought stress [
58,
63]. Priming with ALA also preserves plant photosynthesis in oilseed rape, wheat, and sunflower by suppressing chlorophyll degradation and increasing photosynthetic rate (Pn) during drought stress [
59,
61,
64,
65]. Furthermore, pretreatment with ALA induces the expressions of enzymes involved in the Calvin cycle such as triose-3-phosphate isomerase (TPI) and fructose-1,6-bisphosphate aldolase (FBPA) [
66]. Interestingly, in addition to enhance the drought resistance of plants, ALA priming also improves waterlogging tolerance in Fig (
Ficus carica), with higher levels of antioxidant enzyme activity, photosynthetic efficiency, and root respiration [
67].
3.4. ALA Priming Attenuates UV-B-Induced Damage
Ultraviolet-B (UV-B) radiation is a component of sunlight that induces several plant photomorphogenic responses, including hypocotyl growth inhibition and cotyledon curling [68]. High-intensity UV-B injures plants by damaging DNA, impaired photosynthesis, and cell death, and triggering the accumulation of ROS [69]. Priming with ALA was reported to significantly reduce plant damage from UV-B radiation by promoting photosynthesis, enhancing antioxidant capacity, and improving nitrogen metabolism. As a key precursor of chlorophyll biosynthesis, ALA alleviated the deficiency of chlorophyll biosynthesis during UV-B stress; ALA pretreatment upregulates the expression of genes involved in chlorophyll biosynthesis such as glutamyl-tRNA reductase (HEMA1), Mg-chelatase (CHLH), and protochlorophyllide oxidoreductase (POR) in pigeon pea (Cajanus cajan), thus promoting plant photosynthesis during UV-B stress [8,70]. In addition, ALA priming-increased activities of antioxidant enzymes are essential for lettuce (Lactuca sativa) resistance to UV-B stress [71]. In addition to enzymatic antioxidants, ALA also increases the content of non-enzymatic antioxidants such as flavonoids and phenolics [8]. Under UV-B stress conditions, ALA priming significantly improves the activities of nitrate reductase (NR), nitrite reductase (NiR), glutamine synthetase (GS), and glutamate synthase (GOGAT), and then increases the levels of NO3− and NO2− in the seedlings of pigeon pea [70]. Collectively, ALA priming contributes to UV-B tolerance by regulating photosynthesis, antioxidant, and nitrogen metabolism in plants.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020702