Produced water (PW) is the most significant waste stream generated in the oil and gas industries. The generated PW has the potential to be a useful water source rather than waste. While a variety of technologies can be used for the treatment of PW for reuse, biological-based technologies are an effective and sustainable remediation method. Specifically, microalgae, which are a cost-effective and sustainable process that use nutrients to eliminate organic pollutants from PW during the bioremediation process. In these treatment processes, microalgae grow in PW free of charge, eliminate pollutants, and generate clean water that can be recycled and reused. This helps to reduce CO2 levels in the atmosphere while simultaneously producing biofuels, other useful chemicals, and added-value products.
1. Introduction
Produced water (PW) is comprised of an enormous amount of industrial wastewater (WW) generated from oil and gas extraction [
1]. This water naturally occurs within the oil reservoir and is generated during the extraction stage [
2]. Approximately 250 million barrels of PW are created every day by oil and gas industries, and more than 40% of that is released into the environment [
3], which represents a serious environmental threat. The composition of PW is determined by the geological age, depth, geochemical composition of the area carrying hydrocarbons, the chemical composition of crude oil and natural gas in the zone, and the chemicals introduced during the exploratory process [
4]. There is no constant volume of PW in oil and gas exploration as it is dependent on the geographic location of the field and the geological formation [
5]. The constituents in the PW are toxic organic compounds such as benzene, toluene, ethylbenzene, and xylenes (known as BTEX), inorganic compounds such as heavy metals, total dissolved solids (TDS), chemical additives used during the oil production process, polyaromatic hydrocarbons (PAHs), and other pollutants [
4,
5,
6,
7]. The presence of these components in PW increases its toxicity, creates significant environmental concerns, and reduces the possibility of treating and reusing the water.
Typically, discharging partially treated PW is allowed in specific standard quantities. However, there is a high possibility that over time this water may cause chronic toxicity, which affects the environmental ecosystem [
8,
9]. It is expected that the volume of PW will imminently increase due to the expansion of the oil and gas industry. Thus, it is crucial to find an efficient and sustainable mechanism to treat and utilize the PW.
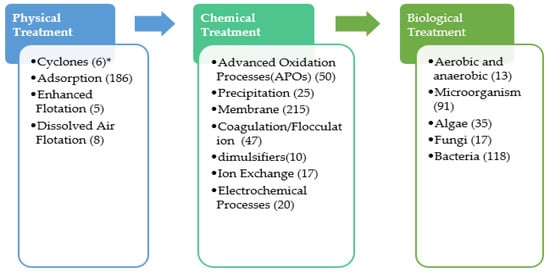
Figure 1 presents the technologies that were studied for the treatment of PW since 2016 [
11,
12,
13,
14].
Figure 1. General PW treatment processes. * Number of published papers since 2016.
In contrast, algal treatments are an effective technique to treat a variety of industrial wastewaters [
15]. Microalgae can be used to treat PW and remediate organic pollutants with the use of specific algal species. Moreover, microalgae treatment processes produce usable biomass for biofuel production and have an additional benefit of CO
2 capturing. In such treatment processes, algal cultures can solve economic and environmental problems while simultaneously producing biofuels and other useful chemicals that reduce CO
2 levels in the atmosphere [
16]. With an increase in water resource demands, PW has been used to maintain freshwater resources, especially in arid regions suffering from freshwater scarcity. Investigations into the use of algae for PW treatment concluded that it is not sufficiently advanced due to a need for large quantities of nutrients, solar radiation, CO
2 supply, freshwater, and an adequate area for the cultivation medium [
17,
18].
3. Produced Water
3.1. Produced Water Generation
Water created as a by-product during the extraction of oil and natural gas is referred to as produced water (PW). This type of water is frequently found in oil and gas reservoirs, occasionally in a zone underneath the hydrocarbons, and in the same zone as the oil and gas. PW is a type of brackish and saline water that is brought to the surface from underground formations [
19]. Typically, oil wells can generate enormous amounts of water together with oil, but gas wells produce water in smaller amounts. In 2018, it was estimated that the production of one U.S. barrel of oil (≈0.16 m
3) was combined with of 3.13 barrels (≈0.50 m
3) of PW [
4]. This demonstrates that the water to oil ratio is approximately around 3:1. Reports indicate that oil fields account for more than 60% of PW generation worldwide [
4].
As previously noted, there is no constant production volume of PW in oil and gas generation, as it is dependent on the geographic location and geological formations [
5]. In 2007, the production of crude oil was 53,463.4
barrel/day and 31,449.1 barrel/day of natural gas from the Sergipe and Alagoas oil field in northeastern Brazil was combined with 207,563.8
barrel/day of PW [
20]. Only 85% of the PW was sent to a treatment plant and the remaining was re-injected into the well to help extend the oil field production lifetime [
24]. For instance, the U.S. oil industry produces the largest amount of PW [
18], with New Mexico as the third-largest oil-producing state in the United States. According to 2019 data, New Mexico produced 1.246 billion barrels of PW [
25]. It was reported that Mississippi Oil and Gas generates 330,026,777 bbl/year of PW during gas production [
26]. Another large producer is Oman, whose daily extracted volume of PW from Nimr Field reaches 5032 bbl/day [
27]. Moreover, Oman’s Nimr field can re-inject 120 million L/d of PW into the ground [
27]. Moreover, in 2020, Qatar’s average production of PW from the offshore North Field produced 26,554 bbl/day [
28]. The average water to gas ratio recorded during natural gas generation from Qatar’s North Field is 1.2 [
28]. These high volumes of PW highlight the urgent need for cost-effective treatment methods. Al-Ghouti et al. [
8] characterized Qatar’s PW from the Natural Gas Field, which has been outlined in
Table 1.
Table 1. Produced water characterization in Qatar from the Natural Gas field [
8].
| Parameter |
Raw Produced Water |
Filtered Water |
| Total organic carbon (mg/L) |
389.1 |
317 |
| Total nitrogen (mg/L) |
35.77 |
27.6 |
| Total phosphorus (μg/L) |
277.78 |
180 |
| Benzene (mg/L) |
21 |
16.1 |
| Toluene (mg/L) |
3.8 |
3.21 |
| Ethylbenzene (mg/L) |
1.22 |
1.05 |
| Xylene (mg/L) |
3.43 |
3.11 |
3.2. Characteristics of Produced Water
Produced water characteristics vary between regions and a specific study for each area should be conducted to investigate the effects of PW discharge on the environment [
8,
18]. Further, PW contains a complex composition of physical and chemical properties, dependent on the geological formation, geographic field [
5], extraction method, and the type of extracted hydrocarbon [
6]. Rahman et al. [
1] detail a list of PW parameters and their typical range. It was observed that the toxicity of the PW generated from gas wells is 10 times greater than the toxicity produced from oil wells [
5]. Given that, special treatment should be taken for PW from oil wells.
The composition of PW from oil fields is summarized in
Table 3. The primary constitutes found in PW are total dissolved solids (TDS), salts, benzene (B), toluene (T), ethylbenzene (E), and xylenes (X) (denoted as BTEX), polyaromatic hydrocarbons (PAHs), oil and grease (O&G). The BTEX are volatile organic compounds that naturally occur in oil and gas wells, including gasoline and natural gas. The BTEX compounds also freely escape into the atmosphere during PW treatment [
31]. Additionally, traces of natural organic and inorganic compounds, phenol, organic acids, and chemical additives added during the drilling process can be found in PW and contribute to its total toxicity [
5].
Table 3. Composition of PW from oil and gas field.
| Composition |
Concentration Range (mg/L) |
References |
| Chemical oxygen demand (COD) |
1220–2600 |
[4,6,8,32,33] |
| Sodium ions (Na+ |
| ) |
0–150,000 |
| Total suspended solids (TSS) |
1.2–1000 |
[4,6,8,20,32,33] |
| Calcium ion (Ca2+ |
| ) |
0–74,000 |
| Total polar compounds |
9.7–600 |
[6,8,34] |
| Boron (B) |
5–95 |
| Total dissolved solids (TDS) |
100–400,000 |
[8,34] |
| Chlorine (Cl−) |
| |
0–270,000 |
| BTEX; benzene (B), toluene (T), ethylbenzene (E), and xylenes (X) |
0.73–24.1 |
[4,32] |
| Magnesium (Mg2+) |
| |
8–6000 |
| Total organic compound (TOC) |
0–1500 |
[4,6,20,32,33] |
| Iron(II) (Fe2+ |
| ) |
0.1–1100 |
| Total oil and grease |
2–565 |
[34] |
| Barium ion (Ba2+) |
| |
0–850 |
| Phenol |
0.009–23 |
[6,32,34] |
| Potassium ion (K+ |
| ) |
24–4300 |
| pH |
4.3–10 |
[4] |
| Strontium ion (Sr+ |
| ) |
0–6250 |
| Total organic acids |
0.001–10,000 |
[4] |
| Aluminium (Al3+ |
| ) |
3–40 |
[4,32] |
| Lead (Pb) |
0.008–0.08 |
| Bicarbonate (HCO−3 |
| ) |
0–15,000 |
[8] |
| Arsenic (As) |
0.002–11 |
| Sulfate (SO2−4 |
| ) |
0–15,000 |
[4,6,8] |
| Manganese (Mn) |
0.004–175 |
| Titanium (Ti) |
0.01–0.7 |
[4,6] |
| Zinc (Zn) |
0.01–35 |
3. Algae-Based Biological Processes
Microalgae are an encouraging technology for the treatment of WW [
47,
48,
49,
50,
51,
52]. For example, microalgae can uptake different constituents from PW and use them as a growth medium. Given that, algal cultures can solve both economic and environmental concerns and simultaneously produce biomass and other useful chemicals [
53,
54]. Establishing a sustainable green technology such as algae for PW treatment, recovery, and reuse contributes to the production of biomass, which can be converted into biofuel [
48,
54,
55,
56,
57]. This conversion helps to eliminate and save natural gas. Moreover, naturally occurring microorganism seeds in PW can sequentially work with algae and increase the removal efficiency of organic matters and dissolved solids. In sequential processes, algae consume CO
2 and produces O
2, which are essential components for the survival of the microorganism.
Table 5 presents the efficiencies of different microalgae strains and their ability to remove organic compounds and nutrients from wastewater. The removal efficiencies reached up to 50%, 65%, and ≥80% for nitrogen compounds, phosphorous, and heavy metals, respectively. Other constituent (i.e., COD and BETX) removals were related to the strain that was used. Algae-based wastewater treatment can also be performed in different systems as outlined in Table 6. Depending on the type of system used (i.e., open vs. closed), different removal efficiencies can be achieved.
Table 5. Efficiencies of microalgae in removing organic compounds and nutrients.
| Microalgae Species |
Type of Nutrients |
Removal Efficiency% |
References |
| Dunaliella salina |
Nitrogen
Phosphorus
heavy metal:
Ni
Zn |
65%
40%
90%
80% |
[63] |
| Nannochloropsis oculata |
Ammonium and Nitrogen
Organic carbon
Iron |
~100%
40%
>90% |
[64] |
| Parachlorella kessleri |
Benzene and Xylenes
Toluene
Ethylbenzene |
40%
63%
30% |
[65] |
Chlorella vulgaris (C.v)
Neochloris oleoabundans (N.o) |
COD by (C.v)
by (N.o)
Ammonia by C.v. and N.o
Phosphorus by C.v. and N.o |
51%, 55% and 80%
63%, 47% and 72%
(70–84%)
(>84%), (>22%) and (<15%) |
[66] |
| Chlorella pyrenoidosa |
Chromium
Nickel |
11.24%
33.89% |
[67] |
Table 6. Microalgae cultivation system in different wastewater.
| Cultivation System |
Algae Species |
Cultivation Condition |
Type of Waste |
Biomass Productivity g/(L.d). |
Organic Removal |
Biofuel Type |
Refs. |
| Closed system (PBRs) |
Scenedesmus acutus (UTEX B72) |
Agriculture-grade urea, triple super phosphate (TSP), pot ash and Sprint 330 (iron chelate) |
Flue gas |
0.15 |
Sulfur, NOx |
|
[68] |
| Closed system 4-L cylindrical photobioreactor (PBR) |
Mixed culture of Chlorella vulgaris, Scenedesmus Obliquus, Botryococcus braunii, Botryococcus sudeticus, and Afrocarpus falcatus |
pH = 7, Temp = 25 °C. |
|
0.15 |
21, 60, and 47% for protein, carbohydrate and DOC, respectively |
|
[69] |
| 500 mL glass flasks |
Dunaliella tertiolecta |
pH—8.1, Temp = 24 °C, f/2 medium |
Real PW |
0.0172 @ salinity 30 gTDS/L to 0.0098 @ 201 gTDS/L |
|
Biodiesel |
[61] |
| 500 mL glass flasks |
Cyanobacterium aponinum, Parachlorella kessleri |
pH—8.1, Temp = 24 °C, f/2 medium |
Real PW |
0.113 * |
|
Biodiesel |
[70] |
| |
Synechococcus sp., Cyanobacterium aponinum and Phormidium sp. |
pH = (6–9), |
BG-11 medium |
NA |
|
Biodiesel |
[71] |
| |
Chlorella sp. and Scenedesmus sp. |
pH = 7.1 |
|
0.115 * |
Chlorella sp.: remove 92% of the TN and 73% of the TOC |
|
[72] |
| |
Dunaliella salina |
Salinity 52.7–63.3 g/L NaCl |
Real produced water |
NA |
Aluminum, barium, copper, magnesium, manganese, nickel, and strontium |
Biodiesel |
[59] |
| Horizontal laminar air flow chamber |
Chlorella pyrenoidosa |
T = 121 °C |
Fogg’s Medium, slant culture |
NA |
|
Biofuel and bioplastic |
[67] |
This entry is adapted from the peer-reviewed paper 10.3390/su14010499