For hybrid electric vehicles, supercapacitors are an attractive technology which, when used in conjunction with the batteries as a hybrid system, could solve the shortcomings of the battery. Supercapacitors would allow hybrid electric vehicles to achieve high efficiency and better power control. Supercapacitors possess very good power density. Besides this, their charge-discharge cycling stability and comparatively reasonable cost make them an incredible energy-storing device. The manufacturing strategy and the major parts like electrodes, current collector, binder, separator, and electrolyte define the performance of a supercapacitor. Among these, electrode materials play an important role when it comes to the performance of supercapacitors. They resolve the charge storage in the device and thus decide the capacitance.
1. Introduction
Among the clean energy portfolio, it is undeniably true that electrochemical energy is one of the most important parts of it. Fuel cells, batteries, and supercapacitors are the major devices working on the electrochemical energy conversion principle.
High specific capacitance, high power density, long life cycle, and very little maintenance have gained supercapacitors much attention as an energy storage device like a battery, and its ability to function as a bridge between capacitor and batteries has made the scientific community focus on the research and development of supercapacitors. One of the major attractive aspects of supercapacitors is that both charging and discharging can be done within a short period of time. Since both battery and capacitor collect and release energy, they may seem similar, but the vital difference between battery and capacitor is how they work contrarily on set-up applications. While batteries deliver better energy density for storage, capacitors provide more speedy charge and discharge capabilities.
Table 1 shows the comparison between batteries and supercapacitors [
1].Batteries are preferred for applications where high energy density is required but with limited power output and requiring long term use of energy. When energy is required to be delivered at high power, capacitors are preferred.
Table 1. Comparison between batteries and supercapacitor [
1].
| Comparison Parameter |
Battery |
Supercapacitor |
| Storage mechanism |
Chemical |
Physical |
| Power limitations |
Reaction kinetics, mass transport |
Electrolyte conductivity |
| Charge rate |
Kinetically limited |
High |
| Energy storage |
High |
Limited |
| Cycle life limitations |
Mechanical stability, chemical reversibility |
Side reactions |
The utilization of electrode materials with more surface area and very slim dielectrics makes supercapacitors accomplish better capacitance and that makes them different from the conventional capacitors. The higher power capabilities and long cycle life compared to batteries makes supercapacitors attractive technology and provide a promising technology to manufacture superior energy storage systems [
2].
Supercapacitors can manage high power rates, which, when compared with batteries, is high, but even though they provide very high power in the same volume compared to batteries [
1], the inability to store a similar quantity of charge as batteries are capable of (three to thirty times lower) is their biggest disadvantage. This is why supercapacitors are used for applications where large energy storage capacity is not required but only high-power bursts are needed. In battery-based energy storage systems, supercapacitors can also be included to decouple the energy and power characteristics of the energy storage systems. This could improve the sizing and lifetime of the system while at the same time attaining the demands in power and energy.
2. Electrode Materials
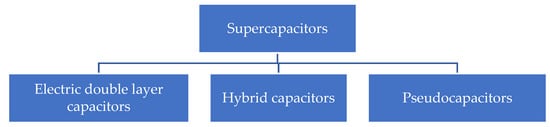
Supercapacitors are divided generally into different types mainly according to the charge storage mechanism.
Figure 1 shows the general classification of the supercapacitors. One is electric double-layer capacitors (EDLCs) and the other is pseudo capacitors (PCs). EDLCs are sometimes also called electrostatic capacitors. The charge storage in EDLCs takes place at the electrode/electrolyte interface through the electrostatic charge absorption mechanism. The most attractive materials for EDLCs have been carbon-based materials, mainly due to their abundance in nature and the high surface area; on the downside, the relatively low specific capacitance is a disadvantage [
9].
Figure 1. Classification of supercapacitors.
The Electric Double Layer Capacitor (EDLC) materials stock up charge mainly in an electrochemical double layer formed on the surface of the electrode but not in its bulk. Therefore, the capacitance mainly depends on the surface area of electrode, which can be accessed by the electrolyte ions. The important factors controlling the electrochemical activity of electrodes are the specific surface area, pore size distribution, pore shape, morphology, conductivity, and surface functionality.
2.1. Carbonaceous Materials
Carbon-based materials are the most commonly used material for various applications in supercapacitors, thanks to their high availability and robust production processes in the industry, which in turn result in the reduced cost [
15]. Among the applications, electrodes based on carbon materials are highly popular. They can be produced in various forms like fibers, nanotubes, and foams from 1D to the 3D structure. Usually, the electrode surface area, manufactured with carbon, is directly proportional to the specific capacitance, but this is not always the case. Some types of carbon will have higher specific capacitance even when they have a lower surface area compared to the electrode with a high specific area [
16].
2.2. Metal-Organic Framework (MOF) Based Electrode Materials
The attention metal-organic framework is receiving as a template for the synthesis of nanocomposites of porous carbon, metal/metal oxides, and porous MOs has gathered momentum recently. In general, pristine MOFs are being used as positive electrodes for supercapacitor devices, whereas MOF-derived carbon is being used as a negative one. Compared to pure carbon-based materials, these MOF-derived carbon materials deliver excellent electrochemical performance owing to their favorable natures like high porosity, high specific surface area, etc. Annealing of MOFs at high temperature under inert atmosphere converts them into carbon, retaining the original MOF template [
115,
116]. The preparation of MOFs has been done by combining the organic and inorganic units through solid chemical bonds.
2.3. Bimetallic Metal-Organic Framework (BMOF)
To enhance the intrinsic properties of MOFs, bimetals are combined with MOF structure as they can favorably introduce high porosity and defects due to the combinational effects between the various types of metals. This enables BMOFs to be used in various applications like electrodes of supercapacitors. Producing heterojunctions with the help of bimetallic metal organic frameworks is going to provide a new way to study the effects between the mixed metal atoms and this is believed to eliminate many limitations faced during the current practical applications of electrode materials. Enhancing the performance of metal organic framework based supercapacitors by combining another metal ion into the framework has been proposed [
136]. The electron’s electrical conductivity between electrolyte surfaces and electrodes is improved considerably as the second metal node is doped in a MOF, which could effectively endorse electronic coupling between the metal node [
137]. Even though BMOF is manufactured from MOF, its morphology is different, but still in most of the cases it holds the crystal structure of the pristine MOF [
136,
137,
138,
139,
140]. As the BMOF possesses improved surface area, porosity, lesser particle size, and better conductivity in comparison to MOF supercapacitors, its capacitance can be elevated. Multiple valance states of the metal species are present in BMOFs inducing higher redox sites, which makes them an interesting material in the supercapacitor electrode research studies [
141].
2.4. Conducting Polymers
Conducting polymers (CPs) enables the faradaic redox reactions and thus helps improve the specific capacitance of the supercapacitors. They are usually seen in composite materials used for the synthesis of supercapacitor electrodes. An improved pseudocapacitance is resultant due to the faradaic redox reactions. The charging of conducting polymers takes place all over the material, whereas only surface is involved for plain carbon electrodes. For the redox reaction, the ions from the electrolyte transfer into the polymer and out of it which results in an improved capacitance and also exhibits reduced cyclability [
158]. Polyaniline (PANI) is one of the examples of this type of polymer which has undergone many studies. Its low cost, better conductivity, and comparatively easier production methods made them an attractive material.
2.5. Transition Metal Oxides
Most of the research based on transition metals for supercapacitors has mainly studied the oxides of transition metals, since pseudocapacitance was discovered in 1971 [
62]. The studies on transition metal dichalcogenides (TMDC) has begun only recently. TMDC also contains oxides like RuO
2. However, as chalcogen is in the 16th group of the periodic table, transition metal dichalcogenides are not metal oxides in reality and that is why the two are labelled separately in most cases. TMDC includes oxides of metals like titanium, molybdenum, sulfides of metals like tungsten, etc. [
161,
162,
163,
164,
165,
166,
167,
168].
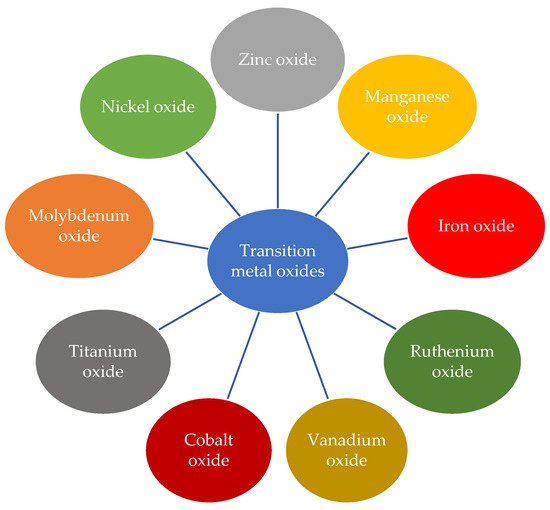
Figure 3 shows some of the most studied transition metal oxides.
Figure 3. Examples of transition metal oxides.
2.6. Transition Metal Nitrides
The exceptional chemical bonding and intrinsic structures of transition metal nitrides allow them to exhibit outstanding conductivity and physiochemical properties and this has gained them great attention for supercapacitor applications. The poor stability in cycling and low conductivity are major hurdles when it comes to the application of metal oxides and conducting polymers as pseudocapacitive materials. This is where metal nitrides come into the picture as a promising alternative. The metal nitrogen bond formation decides the properties of the material. Very good ionic conductivity has been shown by group 1 and group 2 based nitrides whereas nitrides formed from group III and IV, because of their intrinsic covalent bonding, exhibit stiffness and tolerance against high temperatures [
212].
TMN bonding is a combination of metallic, ionic, and covalent bonding. Additionally, the lattices of transition metal nitrides are metallic structures occupied by nitrogen atoms which are disoriented in nature at the interstitial sites [
213]. All these unique characteristics provide them with exceptional properties such as multiple crystal structures and valence states, better electrical conductivity than the transition metal oxides, and also better redox chemistry which in turn results in good electrochemical activities [
214,
215,
216]. Since the demonstration of titanium metal nitride applications in supercapacitors began when molybdenum nitride films were studied to use as a substitute in electrodes of ruthenium oxide [
217], they have become one of the most important materials studied and considered to be promising in energy-related research and there have already been made commendable achievements both in practical and theoretical developments.
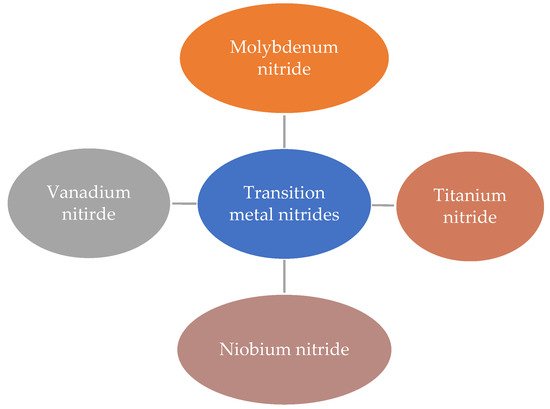
Figure 5 shows some of the promising transition metal nitrides studied for application in high-performance supercapacitors.
Figure 5. Examples of transition metal nitrides.
2.7. Redox Polymers
Redox polymers are another promising material that can be used as electrodes in the manufacturing of a sustainable, affordable, and high-performance supercapacitor. Their mechanical flexibility, better processability, small cost, molecular diversity, and good electrochemical activity has made them an attractive material. These properties make them a better candidate for the practical application of supercapacitors than conventional inorganic materials like metal oxides or carbons [
258]. The supercapacitors based on this material not only generate pseudocapacitance from the reversible redox reaction of electrode materials but also accumulate charge at the electrical double layer. Because of this, a much better specific capacitance and energy density than EDLCs is exhibited by the supercapacitor [
259].
This entry is adapted from the peer-reviewed paper 10.3390/condmat7010006