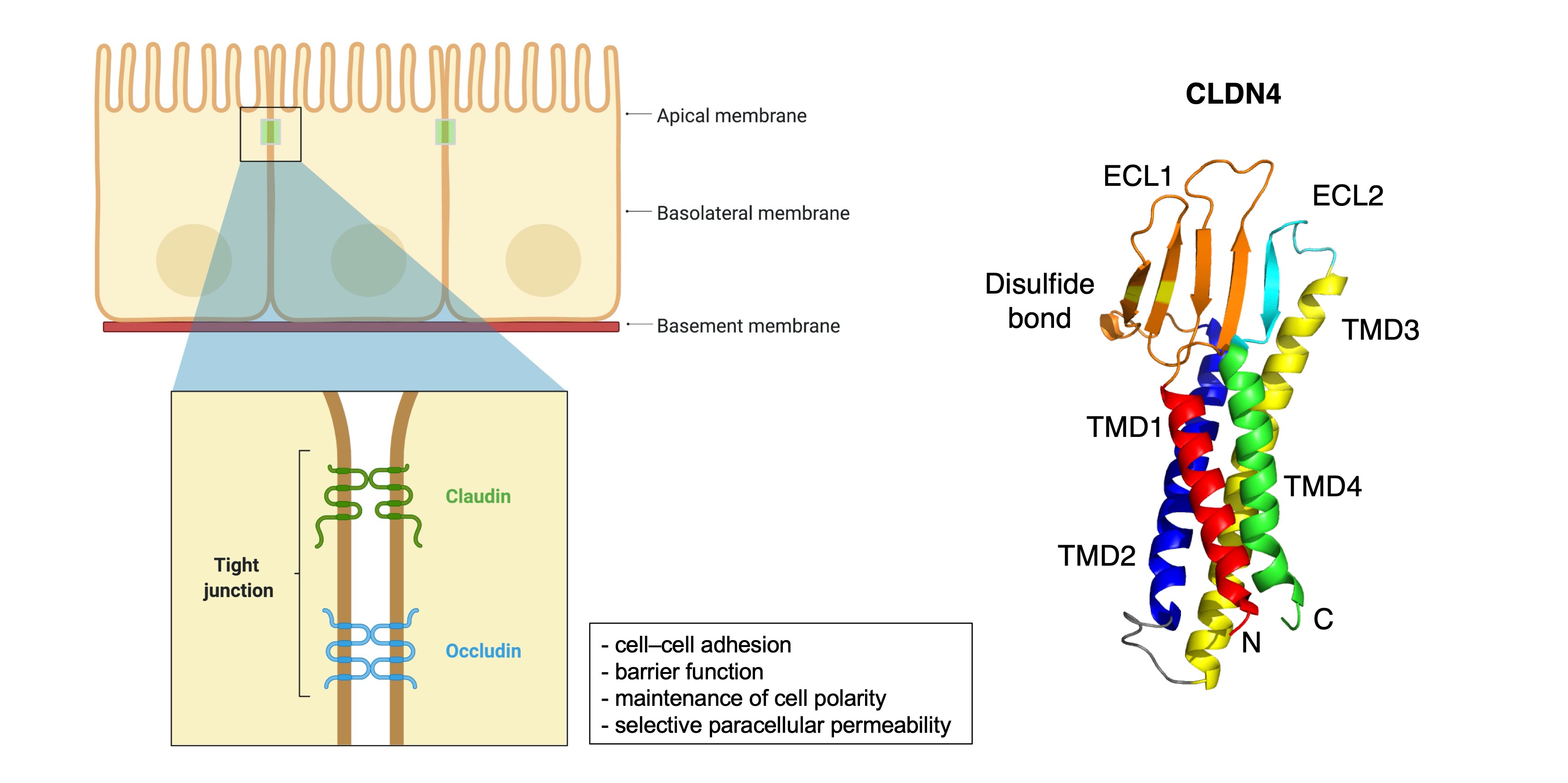
The expression of several CLDNs has been described in GC, including their functions, molecules involved, and clinical significances (Table 1). The following section summarizes new insights and deeper considerations for each CLDN’s expression in GC.
3.1. CLDN18
The CLDN18 protein is the most studied among the CLDNs, since it is specifically expressed in stomach and GC tissue, compared to other tissues or cancers, which makes it a potential therapeutic target. CLDN18 was first identified in 2001 as a downstream target gene of the T/EBP/NKX2.1 homeodomain transcription factor [
36]. The human
CLDN18 gene has two splice variants, which encode two protein isoforms, CLDN18 splice variant 1 (CLDN18.1) and CLDN18 splice variant 2 (CLDN18.2) [
37]. CLDN18.1 is specifically expressed in normal and cancerous lung tissues, and CLDN18.2 is expressed in normal gastric tissues and in tissues of gastric, pancreatic, esophageal, and lung cancers [
37]. In the normal gastric mucosa, CLDN18 is found on the surface of the lobular epithelium, immature cells, and glandular epithelium. In intestinal metaplasia, CLDN18 is absent in metaplastic epithelium [
38].
Table 1. Claudin’s functions, signaling molecules involved, and clinical significances in GC.
| Type of CLDN |
Main Functions, Signaling Molecules Involved, and Clinical Significances in GC |
References |
| CLDN1 |
- correlation with tumor infiltration and metastasis
- Akt, Src, and NF-κB signaling pathway
- poor prognostic factor |
[39,40]
[41,42]
[40,43] |
| CLDN2 |
- CDX2-dependent targeting relationship with CagA produced from H. pylori |
[44] |
| CLDN3 |
- correlation with lymph node metastasis
- important immunosuppressive regulator |
[45]
[46] |
| CLDN4 |
- correlation with lymph node metastasis
- promoting EMT and infiltration of MMP-2 and MMP-9 |
[47]
[48,49,50] |
| CLDN6 |
- cell proliferation and migration/infiltration with YAP1
- high expression was positively correlated with decreased OS |
[51]
[51,52] |
| CLDN7 |
- proliferation in a CagA-and β-catenin-dependent manner
- poor prognostic factor |
[53]
[54] |
| CLDN10 |
- association with metastasis and proliferation |
[55,56] |
| CLDN11 |
- correlation with H. pylori infection and Borrmann classification, not with lymph node metastasis and TNM stage |
[57] |
| CLDN14 |
- correlation with lymph node metastasis |
[58] |
| CLDN17 |
- correlation with lymph node metastasis |
[58] |
| CLDN18 |
- correlation with metastasis (lymph node, peritoneal, bone, and liver metastasis)
- poor prognostic factor
- Wnt, β-catenin, CD44, EFNB/ EPHB receptor signals, and HIPPO signals
- CLDN18-ARHGAP fusion in genomically stable type
- therapeutic target of Claudiximab (IMAB362, Zolbetuximab) |
[59,60]
[61,62,63,64,65,66,67,68]
[54,69,70]
[8]
[10,71,72,73] |
| CLDN23 |
- poor prognostic factor |
[57] |
Downregulation of CLDN18 has been reported to occur during intestinal metaplasia [
74,
75] in the Correa cascade, a multistep and multifactorial process of gastric carcinogenesis [
69,
70]. Notably, the expression of CLDN18 in GCs has different biological functions depending on whether it is up- or downregulated; CLDN18 is also highly expressed in normal gastric tissues, and downregulation of this expression was detected in 57.5% of GCs, especially in 73.7% of intestinal phenotypes [
69]. Similarly, CLDN18 was downregulated in GC compared to the normal mucosa of the surrounding stomach and intestinal metaplasia [
54,
76].
In the relationship between CLDN18 expression and clinicopathological factors in GC, CLDN18 expression was significantly lower in patients with peritoneal metastasis (PM) than those without PM (
p = 0.01). Meanwhile, CLDN18 expression was significantly higher in patients with bone metastasis than in those without bone metastasis (
p = 0.01) [
59]. Another IHC study showed that CLDN18 expression correlated with lymph node metastasis (
p = 0.04), high stage disease (III, IV) (
p = 0.019), and lower incidence of liver metastases (
p = 0.009) [
60]. In addition, a vital relationship existed between the decreased expression of CLDN18 and perineural invasion [
54].
In tissues of early-stage GC removed via endoscopic mucosal resection or endoscopic submucosal resection, the Ki-67 labeling index at the invasive front inversely correlated with the CLDN18 expression level, suggesting that a decrease in CLDN18 expression promotes cancer invasion in early-stage GC [
76]. Furthermore, concerning the relationship between CLDN18 expression and survival, patients without CLDN18 expression reported to have shorter overall survival (OS) than those with CLDN18 expression [
54,
69,
70].
To elucidate the biological significance and carcinogenic mechanism of reduced CLDN18 expression in GC, studies were conducted on GC cell lines in which CLDN18 was knocked down by siRNA and knocked out in mice [
76,
77]. GC cells knocked down by CLDN18 promoted gastric cancer cell proliferation and infiltration compared to the controls [
76]. In CLDN18 knockout mice, CLDN18 was reported to be localized to TJs at the base rather than at the apex of gastric epithelial cells [
77]. Furthermore, in CLDN18 knockout mice, gastric mucosal atrophy and convulsive polypeptide expression alteration (SPEM) occur after paracellular H
+ ion leakage and parietal cell death, and SPEM was suggested as the origin of cancer stem cells [
78].
However, it has also been reported that CLDN18 knockout mice do not progress to cancer while maintaining atrophic gastritis and SPEM status [
79]. In other mouse models, CLDN18 deficiency occurred in the gastric mucosa of
Helicobacter pylori-infected mice [
77]. In contrast, the inactivation of CLDN18 in mice not infected by
H.
pylori promotes the growth of intraepithelial neoplasms that develop into polypoid tumors, β-catenin [
61,
62], CD44 [
63], and EFNB/ EPHB receptor signals [
64,
65]. HIPPO signals [
66,
67] and other vital signaling pathways promote cell proliferation, cancer stem cell development, and tumorigenesis. In GC CLDN18 knockout mice, chronic active gastritis developed at middle age (>40 weeks), and the expression of CCL28, a chemokine with lymphocyte chemo-ventilation activity, was observed. At old age (60 weeks or older), 20–30% of these mice develop gastric tumors, and CXCL5 is expressed. These are multifunctional cytokines with neutrophil-attracting, angiogenic, and EMT-inducing effects. During this process, SPEM cells developed, and the expression of CD44-variants, TLR2, and CXCL5 increased. Some features of gastric tumorigenesis in CLDN18 knockdown mice resemble human carcinogenesis associated with
H.
pylori infection. Wnt1 overexpression in transgenic CLDN18 knockout mice promotes gastric tumorigenesis. This indicates that when gastritis is induced by CLDN18 deficiency, Wnt-dependent gastric tumorigenesis may be triggered [
68].
While CLDN18 downregulation is crucial for GC development, proliferation, infiltration, and EMT, positive expression or upregulation of CLDN18 also plays a biologically important role in GC. In immunohistochemistry (IHC) studies, CLDN18 expression was found in 42.2% of GCs and correlated with the mucin phenotype, EBV status, integrin αvβ5, EpCAM extracellular domain EpEX, and lysozymes [
80]. Bioinformatics analysis of
CLDN18 expression in GC patients using multiple public databases revealed that
CLDN18 expression was higher in the EBV-positive group than in other groups [
81]. Tissue microarray analysis showed high membranous CLDN18 expression in 29.4% of primary cases and 34.1% of metastatic cases. Positive expression of membrane-type CLDN18 was significantly associated with the Lauren diffuse type (
p = 0.009) and EBV-related cancers (
p < 0.001) [
82]. In other IHC studies, CLDN18 was frequently expressed at the primary site, GC. In the metastatic cohort, 88% of GCs were positive for CLDN18 [
83].
The TCGA Research Network [
8] revealed that the
CLDN18–
ARHGAP26/6 translocation was enriched in genomically stable tumors, which was also confirmed in diffuse GC [
84]. In the East Asian GC population,
CLDN18–
ARHGAP26/6 was detected in 3% of all cases [
85]. In other aspects, this unique fusion gene is associated with younger patients and invasive diseases, including lymph node and distant metastases [
86,
87]. Histologically, signet ring cell carcinoma (SRCC), which is based on microscopic characteristics according to the classification by the World Health Organization, belongs to the diffuse type [
88]. The whole-genome sequence of 32 SRCC samples from the eastern Chinese population confirmed that the frequency of
CLDN18–
ARHGAP26/6 fusion was 25%. In the validation cohort, patients with
CLDN18–
ARHGAP26/6 fusion had poorer survival than those without fusion. Moreover, they did not benefit from oxaliplatin/fluoropyrimidine-based chemotherapy [
89]. This is consistent with the chemical drug resistance acquired in GC cells overexpressing
CLDN18–
ARHGAP26.
The subtype of gastric intestinal adenocarcinoma with anastomotic glands has been reported to be frequently associated with poorly differentiated adenocarcinoma components [
90,
91]. In recent years,
RHOA mutations and
CLDN18–
ARHGAP fusions are typically present in adenocarcinomas with anastomotic glands via next-generation sequencer and reverse transcription-PCR [
92]. This characteristic chimeric protein CLDN18–ARHGAP in GC may also be associated with carcinogenesis through functional changes, such as the disappearance of CLDN18 and the acquisition of ARHGAP function (
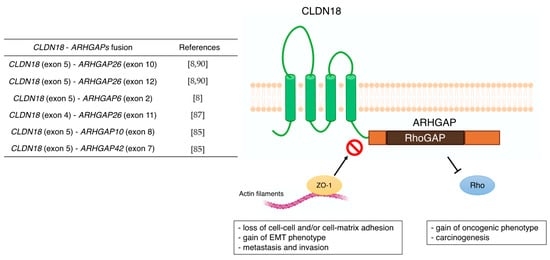
Figure 2). Furthermore, the carboxyl-terminal PDZ-binding motif of CLDN18 interacts with actin-regulating proteins such as RhoA, cadherin, and integrins [
93]. Therefore, loss of the carboxyl-terminal domain of CLDN upon fusion with ARHGAP may prevent actin-regulated proteins from binding to the junctional complex and loosen cell–cell and/or cell–matrix binding. This aberrant ectopic activity of ARHGAP may be involved in carcinogenesis, just as the constitutive inactivation of RhoA leads to the acquisition of a carcinogenic phenotype [
94,
95]. Alternatively, there are reports that mutations in
RHOA are gain-of-function [
84,
96], and further studies are needed concerning the mechanism and effects of this unique fusion of
CLDN18–
ARHGAP in GC. Several cancer cell lines stably expressing
CLDN18–
ARHGAP26 showed a dramatic loss of the epithelial phenotype and long protrusions showing EMT. Fusion-positive cell lines showed loss of barrier properties, reduced cell–cell and cell–extracellular matrix adhesion, delayed wound healing, RhoA inhibition, and acquisition of invasiveness. Thus,
CLDN18–
ARHGAP26 contributes to epithelial degradation, possibly causing H
+ leakage in the stomach, inducing inflammation, and promoting infiltration [
85]. In addition to the
CLDN18 (exon5)-
ARHGAP26 (exon12 or exon10) or
ARHGAP6 (exon2), fusion patterns reported in TCGA cohort [
8] and other reports [
87,
92] have shown
CLDN18 (exon5)–
ARHGAP26 (exon12 or exon10) or
ARHGAP6 (exon2). There are rare cases of
CLDN18 (exon4)–
ARHGAP26 (exon11) [
89],
CLDN18 (exon5)–
ARHGAP10 (exon8), and
CLDN18 (exon5)–
ARHGAP42 (exon7) [
87]. Notably, all these
CLDN18–
ARHGAP fusions share a common RhoGAP domain after gene translocation and are thought to promote carcinogenesis and cancer progression by inactivating Rho.
Figure 2. Previous reports of CLDN18-ARHGAPs and schematic of CLDN18-ARHGAP protein. Created with BioRender.com.
CLDN 18.2 has been reported as a target for therapeutic antibodies [
36,
37,
97,
98,
99]. In normal gastric tissue, CLDN18.2 is contained in TJ supramolecular complexes of gastric mucosal cells, and the epitope of CLDN18.2 has little access to intravenous antibodies [
36,
37,
100]. However, the loss of cell polarity associated with malignancy exposes the epitope of CLDN18.2, making it accessible to bound antibodies, and CLDN18.2, which is maintained in GC and gastric metastases [
37,
54,
101]. Claudiximab (IMAB362, Zolbetuximab) is a novel chimeric IgG1 antibody that is highly specific for CLDN18.2. Claudiximab is derived from a mouse monoclonal antibody and is chimeric with the human IgG1 constant region for clinical application (
Table 2). This novel antibody binds to CLDN18.2 on the surface of cancer cells and stimulates cells and immune effectors that promote antibody-dependent cellular cytotoxicity and complement-dependent cellular cytotoxicity [
71]. Moreover, it can induce apoptosis and suppress cell proliferation. Combination therapy with Claudiximab and chemotherapy may promote T-cell infiltration and induce inflammatory cytokines [
32].
Table 2. Clinical trials associated with IMAB362.
CLDN18.2-positive metastatic, refractory patients were enrolled in a phase I single-dose escalation study of IMAB362 with gastric or esophagogastric junction cancer. Three patients were enrolled in each of the five dose cohorts. In this study, IMAB362 was generally well tolerated at all dose levels; however, the most common adverse event was nausea and vomiting. No dose-limiting toxicity was observed within four weeks. Most patients progressed 4–5 weeks after a single intravenous dose of IMAB362; however, one patient in the 600 mg/m
2 group was stable for approximately 2 months after administration. Based on the pharmacokinetic profile and preclinical dose–response data obtained in this study, a dose of 300–600 mg/m
2 was recommended in a phase II multidrug study [
71].
Another phase I study, PILOT, included IMAB362 plus zoledronic acid (ZA) and a low-to-medium dose interleukin 2 (IL-2) in patients with advanced GC and esophagogastric junction adenocarcinoma. The safety and efficacy of this combination was evaluated (NCT01671774) [
72]. Twenty-eight patients were enrolled, with IMAB362 and ZA in Group 1; IMAB362, ZA, and IL-2 (1 × 10
6 IU, sc) in Group 2; IMAB362, ZA, and IL-2 in Group 3 (3 × 10
6 IU, sc); and IMAB362 alone in Group 4. Among the 20 patients, 11 gained disease control (one with an unidentified partial response and 10 with disease stability). The median progression-free survival was 12.7 weeks, and the median OS was 40 weeks. Grade 1–3 nausea and vomiting were predominant adverse events associated with IMAB362. The PILOT trial revealed that the combination therapy of IMAB and ZA/IL-2 had antitumor effects and was well tolerated.
The multicenter, phase IIa “MONO” study [
9], which follows the phase 1 study “PILOT”, was effective in administering IMAB362 multiple times as monotherapy in patients with GC or lower esophageal cancer [
72]. This was done with the aim of establishing effectiveness and safety. Forty-four patients participated in this study, and data on the antitumor effect of 43 patients were obtained, with an objective response rate (ORR) of 4 (9%) and a clinical efficacy rate (ORR + SD) of 23%. Adverse events occurred in 81.5% (44/54) of patients, with nausea (63%), vomiting (57%), and fatigue (43%). Notably, patients who underwent total gastrectomy had a lower frequency of severe gastrointestinal adverse events (AEs) than those who did not.
The randomized phase IIb study of IMAB362 plus anticancer drug, FAST study, were first-line treatment for patients with advanced gastric–esophageal junction or esophageal adenocarcinoma expressing CLDN 18.2, compared to epirubicin + oxaliplatin + capecitabine (EOX). This study evaluated the efficacy, safety, and tolerability of IMAB362 in combination with EOX [
10]. Patients received EOX (
n = 84) every 3 weeks or received IMB362 + EOX (
n = 77) as an initial treatment. After enrollment, IMAB362 + EOX increased to 1000 mg/m
2 (
n = 85) as an exploratory case group. Progression-free survival (hazard ratio (HR) = 0.44, 95% confidence interval (CI) = 0.29–0.67,
p < 0.0005) and OS (HR = 0.55, 95% CI = 0.39–0.77,
p < 0.0005) in the entire population were extremely good with IMAB362 + EOX (arm2) compared to EOX alone (arm1). Most AEs associated with IMAB362 + EOX were grades 1–2. AEs with grade ≥3 did not show an overall increase compared to EOX alone. These results showed that treatment with IMAB362 targeting CLDN18.2 for patients with GC is an effective and safe treatment. Alternatively, a phase 3 trial, GLOW (NCT03653507), comparing IMAB362 + CAPOX with placebo + CAPOX is underway as a first-line treatment for patients with gastroesophageal junction adenocarcinoma [
73].
Moreover, recently, new antibody drugs that can be expected to have additional therapeutic effects have been developed by modifying existing antibody drugs. This study succeeded in developing a humanized CLDN18.2 specific single-chain fragment variable (scFv) [
102]. Subsequently, CLDN18.2 specific CAR T cells were developed using scFv as a targeting component. CLDN 18.2-specific CAR T cells containing the CD28 costimulatory domain suppressed tumor growth in xenograft mouse models of cancer cell lines. When CAR T cells were administered to a xenograft (PDX) model from a CLDN18.2-positive patient with GC, a partial or complete tumor response was observed. CAR T cells bind well in vivo and efficiently infiltrate tumor tissues. CLDN18.2 CAR T cells could lyse target cancer cells expressing mouse CLDN18.2; however, it had no apparent adverse effects on normal organs, including gastric tissue. In addition, antibody drug conjugates (ADCs) and CD3 bispecific antibodies are being developed as new targeted antibody therapies. Zhu et al. conducted efficacy and preliminary toxicity studies of CLDN18.2 target antibodies via ADC and CD3-bispecific antibodies and their potential therapeutic molecules, with anti-hCLDN18.2 ADC, CD3-bispecific, and diabody. KATO-III/hCLDN18.2 showed in vitro cytotoxicity and suppressed tumor growth in xenograft tumors derived from gastric patients. In a preliminary assessment of tolerability, anti-CLDN18.2 diabodies showed no toxicity in the stomach of NSG mice 4 weeks after dosing [
103]. Given these findings, targeting CLDN18.2 with ADC or a bispecific modality may be a new therapeutic approach for the treatment of GC. To date, most IMAB362 studies employed IHC (CLAUDETECT 18.2VR kit) to assess CLDN18.2 expression in patients with GC. However, given that 92% of the protein sequence of CLDN18.1 is highly consistent with CLDN18.2, the search for more specific CLDN18.2 antibodies remains a major challenge. As a new approach, the CLDN18.2 molecular beacon (MB) with a stem-loop hairpin structure was reported for the detection of CLDN18.2 in blood samples. This MB rapidly recognized the RNA of CLDN18.2 [
104] and was successfully applied to the circulating tumor cell (CTC) assay. This new method of detecting CLDN18.2 RNA in CTCs may be a new approach for identifying potential patients with CLDN 18.2 target drugs.