Plant–microbe interactions range from symbiotic to pathogenic; in the symbiotic relationship, microbes are called ‘endophytes’. Endophytes are conventionally known as microbes existing in all plant endospheric tissues (roots, shoots, fruits, leaves, flowers, seeds, etc.) without causing harmful consequences to the host plant. These microorganisms are usually more abundant in roots and they can be transferred horizontally and vertically. Particularly, endophytic fungi constitute an extremely large community, reaching up to three million species worldwide. These eukaryotic organisms are known to harbor a large variety of secondary metabolites valuable to mankind, plants and the environment. They constitute an excellent substitute for exploring whole plants, thereby gaining time, facilitating the process of isolation and protecting the ecosystem. The scientific community has approved the excellent roles of the fungal bioactive compounds in several vital fields including medicine, pharmacy, agriculture, industry and bioremediation.
- fungal endophytes
- secondary metabolites
- extracellular enzymes
- biotechnological applications
1. History of Fungal Production of Secondary Metabolites
2. Processes of Fungal Secondary Metabolite Production
3. Biotechnological Applications of Secondary Metabolites Produced by Endophytic Fungi
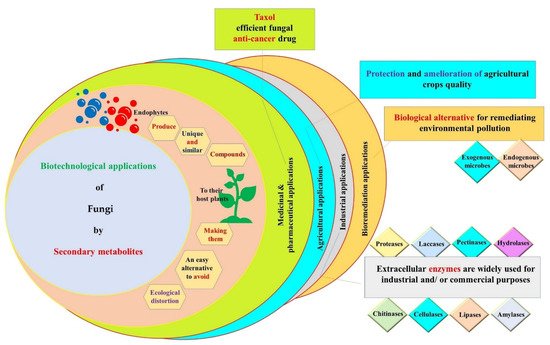
3.1. Medicinal and Pharmaceutical Applications
3.2. Agricultural Applications
3.3. Industrial Applications
3.4. Bioremediation Applications
This entry is adapted from the peer-reviewed paper 10.3390/f12121784
References
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26.
- Kusari, S.; Spiteller, M. Metabolomics of endophytic fungi producing associated plant secondary metabolites: Progress, challenges and opportunities. Metabolomics 2012, 10, 241–266.
- Hou, X.-M.; Wang, C.-Y.; Gerwick, W.H.; Shao, C.-L. Marine natural products as potential anti-tubercular agents. Eur. J. Med. Chem. 2019, 165, 273–292.
- Skellam, E. Strategies for engineering natural product biosynthesis in fungi. Trends Biotechnol. 2019, 37, 416–427.
- Stekel, D. First report of antimicrobial resistance pre-dates penicillin. Nature 2018, 562, 192.
- Ibrar, M.; Ullah, M.W.; Manan, S.; Farooq, U.; Rafiq, M.; Hasan, F. Fungi from the extremes of life: An untapped treasure for bioactive compounds. Appl. Microbiol. Biotechnol. 2020, 104, 2777–2801.
- Manganyi, M.C.; Ateba, C.N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 2020, 8, 1934.
- Newman, D.J.; Cragg, G.M. Endophytic and epiphytic microbes as “sources” of bioactive agents. Front. Chem. 2015, 3, 34.
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40.
- Kusari, S.; Singh, S.; Jayabaskaran, C. Rethinking production of Taxol® (paclitaxel) using endophyte biotechnology. Trends Biotechnol. 2014, 32, 304–311.
- Torres-Mendoza, D.; Ortega, H.E.; Cubilla-Rios, L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. J. Fungi 2020, 6, 58.
- Ortega, H.E.; Torres-Mendoza, D.; Cubilla-Rios, L. Patents on endophytic fungi for agriculture and bio-and phytoremediation applications. Microorganisms 2020, 8, 1237.
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228.
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448.
- Tawfike, A.F.; Romli, M.; Clements, C.; Abbott, G.; Young, L.; Schumacher, M.; Diederich, M.; Farage, M.; Edrada-Ebela, R. Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. J. Chromatogr. B 2019, 1106–1107, 71–83.
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.-M.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207.
- Fernandes, E.G.; Pereira, O.L.; da Silva, C.C.; Bento, C.B.P.; de Queiroz, M.V. Diversity of endophytic fungi in Glycine max. Microbiol. Res. 2015, 181, 84–92.
- Shen, W.; Mao, H.; Huang, Q.; Dong, J. Benzenediol lactones: A class of fungal metabolites with diverse structural features and biological activities. Eur. J. Med. Chem. 2015, 97, 747–777.
- Nielsen, J.C.; Nielsen, J. Development of fungal cell factories for the production of secondary metabolites: Linking genomics and metabolism. Synth. Syst. Biotechnol. 2017, 2, 5–12.
- Martelli, G.; Giacomini, D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 2018, 158, 91–105.
- Adeleke, B.S.; Babalola, O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: A review. J. Fungi. 2021, 7, 147.
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180.
- El Sayed, A.S.A.; Sayed, M.T.; Rady, A.M.; Zein, N.; Enan, G.; Shindia, A.; El-Hefnawy, S.; Sitohy, M.; Sitohy, B. Exploiting the Biosynthetic Potency of Taxol from Fungal Endophytes of Conifers Plants; Genome Mining and Metabolic Manipulation. Molecules 2020, 25, 3000.
- Guo, Z.; Zou, Z.-M. Discovery of New Secondary Metabolites by Epigenetic Regulation and NMR Comparison from the Plant Endophytic Fungus Monosporascus eutypoides. Molecules 2020, 25, 4192.
- Kumar, G.; Chandra, P.; Choudhary, M. Endophytic fungi: A potential source of bioactive compounds. Chem. Sci. Rev. Lett. 2017, 6, 2373–2381.
- Akinola, S.A.; Babalola, O.O. The fungal and archaeal community within plant rhizosphere: A review on their contribution to crop safety. J. Plant Nutr. 2021, 44, 600–618.
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 2020, 11, 852.
- Yan, L.; Zhao, H.; Zhao, X.; Xu, X.; Di, Y.; Jiang, C.; Shi, J.; Shao, D.; Huang, Q.; Yang, H.; et al. Production of bioproducts by endophytic fungi: Chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2018, 102, 6279–6298.
- Savi, D.C.; Aluizio, R.; Glienke, C. Brazilian plants: An unexplored source of endophytes as producers of active metabolites. Planta Med. 2019, 85, 619–636.
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053.
- Kraupner, N.; Ebmeyer, S.; Bengtsson-Palme, J.; Fick, J.; Kristiansson, E.; Flach, C.-F.; Larsson, D.G.J. Selective concentration for ciprofloxacin resistance in Escherichia coli grown in complex aquatic bacterial biofilms. Environ. Int. 2018, 116, 255–268.
- Palanichamy, P.; Krishnamoorthy, G.; Kannan, S.; Marudhamuthu, M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt J. Basic Appl. Sci. 2018, 5, 303–312.
- Manganyi, M.C.; Tchatchouang, C.-D.K.; Regnier, T.; Bezuidenhout, C.C.; Ateba, C.N. Bioactive compound produced by endophytic fungi isolated from Pelargonium sidoides against selected bacteria of clinical importance. Mycobiology 2019, 47, 335–339.
- Tewari, D.; Rawat, P.; Singh, P.K. Adverse drug reactions of anticancer drugs derived from natural sources. Food Chem. Toxicol. 2019, 123, 522–535.
- Chen, L.; Zhang, Q.-Y.; Jia, M.; Ming, Q.-L.; Yue, W.; Rahman, K.; Qin, L.-P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016, 42, 454–473.
- Li, S.-J.; Zhang, X.; Wang, X.-H.; Zhao, C.-Q. Novel natural compounds from endophytic fungi with anticancer activity. Eur. J. Med. Chem. 2018, 156, 316–343.
- Mishra, V.K.; Passari, A.K.; Chandra, P.; Leo, V.V.; Kumar, B.; Uthandi, S.; Thankappan, S.; Gupta, V.K.; Singh, B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PLoS ONE 2017, 12, e0186234.
- Raekiansyah, M.; Mori, M.; Nonaka, K.; Agoh, M.; Shiomi, K.; Matsumoto, A.; Morita, K. Identification of novel antiviral of fungus-derived brefeldin A against dengue viruses. Trop. Med. Health 2017, 45, 32.
- Schön, T.; Miotto, P.; Köser, C.U.; Viveiros, M.; Böttger, E.; Cambau, E. Mycobacterium tuberculosis drug-resistance testing: Challenges, recent developments and perspectives. Clin. Microbiol. Infect. 2017, 23, 154–160.
- Manganyi, M.C.; Regnier, T.; Kumar, A.; Bezuidenhout, C.C.; Ateba, C.N. Biodiversity and antibacterial screening of endophytic fungi isolated from Pelargonium sidoides. S. Afr. J. Bot. 2018, 116, 192–199.
- Uzma, F.; Mohan, C.D.; Hashem, A.; Konappa, N.M.; Rangappa, S.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.K.; Siddaiah, C.N.; et al. Endophytic fungi—Alternative sources of cytotoxic compounds: A review. Front. Pharmacol. 2018, 9, 309.
- Kouipou Toghueo, R.M.; Boyom, F.F. Endophytic fungi from Terminalia species: A comprehensive review. J. Fungi 2019, 5, 43.
- Nalin Rathnayake, G.R.; Savitri Kumar, N.; Jayasinghe, L.; Araya, H.; Fujimoto, Y. Secondary metabolites produced by an endophytic fungus Pestalotiopsis microspora. Nat. Prod. Bioprospect. 2019, 9, 411–417.
- Ambele, C.F.; Ekesi, S.; Bisseleua, H.D.; Babalola, O.O.; Khamis, F.M.; Djuideu, C.T.; Akutse, K.S. Entomopathogenic fungi as endophytes for biological control of subterranean termite pests attacking cocoa seedlings. J. Fungi 2020, 6, 126.
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs. tree pathogens and pests: Can they be used as biological control agents to improve tree health? Eur. J. Plant Pathol. 2019, 155, 711–729.
- Qader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and antibiofilm activities of the fungal metabolites isolated from the marine endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232.
- Bacon, C.W.; White, J.F. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 2016, 68, 87–98.
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340.
- Slama, H.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium antagonistic bacteria from contrasting niches designated the endophyte Bacillus halotolerans as plant warden against Fusarium. Front. Microbiol. 2019, 9, 3236.
- Slama, H.; Triki, M.; Chenari Bouket, A.; Mefteh, F.B.; Alenezi, F.N.; Luptakova, L.; Cherif-Silini, H.; Vallat, A.; Oszako, T.; Gharsallah, N.; et al. Screening of the high-rhizosphere competent Limoniastrum monopetalum’ culturable endophyte microbiota allows the recovery of multifaceted and versatile biocontrol agents. Microorganisms 2019, 7, 249.
- Slama, H.; Cherif-Silini, H.; Chenari Bouket, A.; Silini, A.; Alenezi, F.N.; Luptakova, L.; Vallat, A.; Belbahri, L. Biotechnology and bioinformatics of endophytes in biocontrol, bioremediation, and plant growth promotion. In Endophytes: Mineral Nutrient Management, Volume 3. Sustainable Development and Biodiversity; Maheshwari, D.K., Dheeman, S., Eds.; Springer: Cham, Switzerland, 2021; Volume 26, pp. 181–205.
- Khiralla, A.; Spina, R.; Yagi, S.; Mohamed, I.; Laurain-Mattar, D. Endophytic fungi: Occurrence, classification, function and natural products. In Endophytic Fungi: Diversity, Characterization and Biocontrol; Nova Publishers: New York, NY, USA, 2016; pp. 1–19.
- Liu, T.; Greenslade, A.; Yang, S. Levels of rhizome endophytic fungi fluctuate in Paris polyphylla var. yunnanensis as plants age. Plant Divers. 2017, 39, 60–64.
- Latz, M.A.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567.
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50.
- Cheffi, M.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. root endophyte Bacillus velezensis OEE1 counteracts oomycete and fungal harmful pathogens and harbours a large repertoire of secreted and volatile metabolites and beneficial functional genes. Microorganisms 2019, 7, 314.
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931.
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Gopalan, V. Fungal endophytes: An untapped source of biocatalysts. Fungal Divers. 2012, 54, 19–30.
- Mefteh, F.B.; Frikha, F.; Daoud, A.; Chenari Bouket, A.; Luptakova, L.; Alenezi, F.N.; Al-Anzi, B.S.; Oszako, T.; Gharsallah, N.; Belbahri, L. Response surface methodology optimization of an acidic protease produced by Penicillium bilaiae isolate TDPEF30, a newly recovered endophytic fungus from healthy roots of date palm trees (Phoenix dactylifera L.). Microorganisms 2019, 7, 74.
- Jagannath, S.; Konappa, N.; Lokesh, A.; Dasegowda, T.; Udayashankar, A.C.; Chowdappa, S.; Cheluviah, M.; Satapute, P.; Jogaiah, S. Bioactive compounds guided diversity of endophytic fungi from Baliospermum montanum and their potential extracellular enzymes. Anal. Biochem. 2021, 614, 114024.
- Traving, S.J.; Thygesen, U.H.; Riemann, L.; Stedmon, C.A. A model of extracellular enzymes in free-living microbes: Which strategy pays off? Appl. Environ. Microbiol. 2015, 81, 7385.
- Yadav, A.N.; Kumar, R.; Kumar, S.; Kumar, V.; Sugitha, T.; Singh, B.; Chauahan, V.S.; Dhaliwal, H.S.; Saxena, A.K. Beneficial microbiomes: Biodiversity and potential biotechnological applications for sustainable agriculture and human health. J. Appl. Biol. Biotechnol. 2017, 5, 45–57.
- Mandal, S.; Banerjee, D. Proteases from endophytic fungi with potential industrial applications. In Recent Advancement in White Biotechnology Through Fungi. Fungal Biology; Yadav, A., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 319–359.
- Shubha, J.; Srinivas, C. Diversity and extracellular enzymes of endophytic fungi associated with Cymbidium aloifolium L. Afr. J. Biotechnol. 2017, 16, 2248–2258.
- Srilakshmi, J.; Madhavi, J.; Lavanya, S.; Ammani, K. Commercial Potential of Fungal Protease: Past, Present and Future Prospects. J. Pharmaceut. Chem. Biol. Sci. 2015, 2, 218–234.
- Sahay, H.; Yadav, A.N.; Singh, A.K.; Singh, S.; Kaushik, R.; Saxena, A.K. Hot springs of Indian Himalayas: Potential sources of microbial diversity and thermostable hydrolytic enzymes. 3 Biotech 2017, 7, 118.
- Rajput, K.; Chanyal, S.; Agrawal, P.K. Optimization of protease production by endophytic fungus, Alternaria alternata isolated from gymnosperm tree Cupressus torulosa D Don. World J. Pharm. Pharmaceut. Sci. 2016, 5, 1034–1054.
- dos Santos Aguilar, J.G.; Sato, H.H. Microbial proteases: Production and application in obtaining protein hydrolysates. Food Res. Int. 2018, 103, 253–262.
- Yadav, R.; Singh, A.V.; Joshi, S.; Kumar, M. Antifungal and enzyme activity of endophytic fungi isolated from Ocimum sanctum and Aloe vera. Afr. J. Microbiol. Res. 2015, 9, 1783–1788.
- Archna, S.; Priyank, V.; Nath, Y.A.; Kumar, S.A. Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res. J. Biotechnol. 2015, 10, 4.
- Thomas, L.; Joseph, A.; Singhania, R.R.; Patel, A.K.; Pandey, A. Industrial enzymes: Xylanases. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 127–148.
- Singh, R.V.; Sambyal, K.; Negi, A.; Sonwani, S.; Mahajan, R. Chitinases production: A robust enzyme and its industrial applications. Biocatal. Biotransfor. 2021, 39, 161–189.
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259.
- Le, B.; Yang, S.H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 144.
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543.
- Gomaa, E.Z. Microbial chitinases: Properties, enhancement and potential applications. Protoplasma 2021, 258, 695–710.
- Mathew, G.M.; Madhavan, A.; Arun, K.B.; Sindhu, R.; Binod, P.; Singhania, R.R.; Sukumaran, R.K.; Pandey, A. Thermophilic chitinases: Structural, functional and engineering attributes for industrial applications. Appl. Biochem. Biotechnol. 2021, 193, 142–164.
- Makky, E.A.; Yusoff, M.M. Bioeconomy: Pectinases purification and application of fermented waste from Thermomyces lanuginosus. J. Med. Bioeng. 2015, 4, 76–80.
- Garg, G.; Singh, A.; Kaur, A.; Singh, R.; Kaur, J.; Mahajan, R. Microbial pectinases: An ecofriendly tool of nature for industries. 3 Biotech 2016, 6, 47.
- Pušić, T.; Tarbuk, A.; Dekanić, T. Bio-innovation in cotton fabric scouring-acid and neutral pectinases. Fibres Text. East. Eur. 2015, 109, 98–103.
- Ghanbarzadeh, B.; Ataeefard, M.; Etezad, S.M.; Mahdavi, S. Enzymatic deinking of office waste printed paper: Optimization via response surface methodology. Biomass. Conv. Bioref. 2021.
- Singh, A.; Kaur, A.; Dua, A.; Mahajan, R. An efficient and improved methodology for the screening of industrially valuable xylano-pectino-cellulolytic microbes. Enzyme Res. 2015, 2015, 725281.
- Oliveira, A.C.D.; Watanabe, F.M.F.; Vargas, J.V.C.; Rodrigues, M.L.F.; Mariano, A.B. Production of methyl oleate with a lipase from an endophytic yeast isolated from castor leaves. Biocatal. Agric. Biotechnol. 2012, 1, 295–300.
- Gopinath, S.C.; Anbu, P.; Lakshmipriya, T.; Hilda, A. Strategies to characterize fungal lipases for applications in medicine and dairy industry. BioMed Res. Int. 2013, 2013, 154549.
- Kaur, R.; Saxena, A.; Sangwan, P.; Yadav, A.N.; Kumar, V.; Dhaliwal, H.S. Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nusantara Biosci. 2017, 9, 68–76.
- Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Prasad, R.; Saxena, A.K. Microbiome in crops: Diversity, distribution, and potential role in crop improvement. In Crop Improvement through Microbial Biotechnology; Prasad, R., Gill, S.S., Tuteja, N., Eds.; Elsevier: Noita, India, 2018; pp. 305–332.
- Toghueo, R.M.K.; Zabalgogeazcoa, I.; de Aldana, B.V.; Boyom, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S. Afr. J. Bot. 2017, 109, 146–153.
- Nandy, S.; Das, T.; Tudu, C.K.; Pandey, D.K.; Dey, A.; Ray, P. Fungal endophytes: Futuristic tool in recent research area of phytoremediation. S. Afr. J. Bot. 2020, 134, 285–295.
- Pietro-Souza, W.; de Campos Pereira, F.; Mello, I.S.; Stachack, F.F.F.; Terezo, A.J.; da Cunha, C.N.; White, J.F.; Li, H.; Soares, M.A. Mercury resistance and bioremediation mediated by endophytic fungi. Chemosphere 2020, 240, 124874.
- Zahoor, M.; Irshad, M.; Rahman, H.; Qasim, M.; Afridi, S.G.; Qadir, M.; Hussain, A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Saf. 2017, 142, 139–149.
- Ripa, F.A.; Cao, W.; Tong, S.; Sun, J. Assessment of plant growth promoting and abiotic stress tolerance properties of wheat endophytic fungi. BioMed Res. Int. 2019.
- Bier, M.C.J.; Medeiros, A.B.P.; Soccol, C.R. Biotransformation of limonene by an endophytic fungus using synthetic and orange residue-based media. Fungal Biol. 2017, 121, 137–144.
- Krishnamurthy, Y.L.; Naik, B.S. Endophytic Fungi Bioremediation. In Endophytes: Crop Productivity and Protection: Volume 2 Sustainable Development and Biodiversity; Maheshwari, D.K., Annapurna, K., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–60.
- He, W.; Megharaj, M.; Wu, C.-Y.; Subashchandrabose, S.R.; Dai, C.-C. Endophyte-assisted phytoremediation: Mechanisms and current application strategies for soil mixed pollutants. Crit. Rev. Biotechnol. 2020, 40, 31–45.
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579.
- Rana, K.L.; Kour, D.; Sheikh, I.; Dhiman, A.; Yadav, N.; Yadav, A.N.; Rastegari, A.A.; Singh, K.; Saxena, A.K. Endophytic fungi: Biodiversity, ecological significance, and potential industrial applications. In Recent Advancement in White Biotechnology Through Fungi: Volume 1: Diversity and Enzymes Perspectives Fungal Biology; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–62.
- Deng, Z.; Cao, L. Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere 2017, 168, 1100–1106.
