Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Respiratory System
|
Engineering, Biomedical
|
Radiology, Nuclear Medicine & Medical Imaging
COPD is a progressive lung disease described as accelerated lung aging. The aging, in addition to environmental exposures, increase inflammatory–oxidative stress and cellular senescence, resulting in irreversible lung disease progression from mild to severe emphysema.
- chronic obstructive pulmonary disease
- COPD
- lung
1. Background
Chronic obstructive pulmonary disease (COPD) is characterized by airway obstruction and airflow limitation caused by damage to the alveoli (emphysema) that is worsened by recurring episodes of infections or acute exacerbations (AE-COPD). AE-COPD amplifies inherent inflammatory–oxidative stress responses and impairs airway defense and repair mechanisms, leading to the progressive decline in lung function [1][2][3]. Briefly, inflammation of the airway (bronchitis), which is influenced by environmental, age-related, and/or genetic risk factors, initiates COPD pathogenesis and emphysema progression. There are varying levels of COPD severity that are classified as Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages based on the extent of emphysema and lung function impairment [1]. The primary risk factor for COPD pathogenesis is exposure to harmful particles or gases, such as those found in cigarette or biomass smoke. Thus, cigarette smokers are known to have COPD-related respiratory dysfunctions, limited airflow, and higher prevalence of death [4][5][6]. Similarly, harmful dusts, chemicals, and air pollutants present in the environment or biomass smoke have also been found to increase inflammation of the airways [7][8][9], where repeated exposure can initiate emphysema. Additionally, comorbidities can further accelerate COPD pathogenesis. As an example, those with a history of asthma were found to have a 10- to 30-fold increased risk of developing COPD [10].
Moreover, hereditary factors, such as alpha-1 antitrypsin deficiency (AATD), found in approximately 5% of subjects with COPD, predisposes the subject to develop emphysema. Briefly, alpha-1 antitrypsin is a serine protease inhibitor primarily produced in the liver to protect tissues from damage due to infection and resulting inflammation, where the absence of AAT and/or presence of recurring respiratory exacerbations in these subjects initiates lung tissue damage due to underlying inflammation, leading to COPD-emphysema pathogenesis [1][11]. It is noteworthy that all subjects with AATD do not develop COPD, highlighting the requirement of a trigger such as first- or second-hand smoke, infection, other toxic/inflammatory agents, etc. Additionally, while studying COPD from an epidemiological perspective, the Boston Early-Onset COPD study demonstrated that smokers that are first-degree relatives of subjects with severe, early-onset COPD had a threefold risk of airflow limitation compared to smokers without family history of COPD [12], verifying the role of genetic predisposition in COPD pathogenesis. Furthermore, deficiencies in lung growth or development are also linked to increased exposure to environmental and/or genetic risk factors, specifically, individuals who have reduced maximal attained lung function have limited airflow, leading to the development of COPD over time.
2. Emerging COPD Diagnostics for Real Time Lung Function Assessment
Significant strides in COPD diagnosis have been made with emerging novel diagnostics capable of early diagnosis and/or real time assessment of disease progression. These techniques include FOT/IOS, PAT, XPC, UCT, and EIT, each with unique advantage’s and/or limitations as shown in Figure 1. The corresponding artificial intelligence (AI) and validation software have also been developed as add-on utilities to achieve robustness and automation for overcoming current limitations for clinical bedside translation.
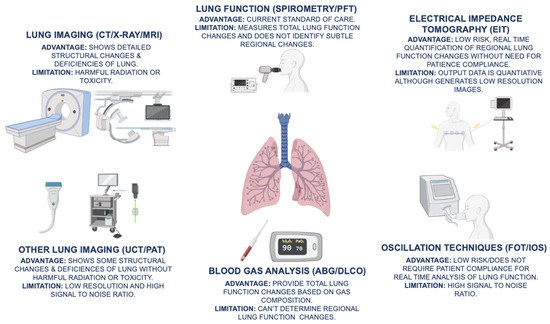
Figure 1. The advantages and limitations of current COPD (chronic obstructive pulmonary disease) diagnostics, which include spirometry/PFT (pulmonary function testing), ABG (arterial blood gas), DLCO (diffusing capacity for carbon monoxide), and lung imaging modalities, such as CT (computed tomography), X-ray, and MRI (magnetic resonance imaging), are illustrated. In addition, emerging novel diagnostic techniques, such as EIT (electrical impedance tomography), FOT (forced oscillation technique), IOS (impulse oscillometry system), UCT (ultrasound computed tomography), and PAT (photoacoustic tomography), provide non-invasive, real-time assessment of changes in lung function.
2.1. X-ray Phase Contrast Imaging and Tomography for Functional Lung Imaging
X-ray phase contrast (XPC) utilizes the diffraction of the X-rays to measure the shift in the phase after passing through the tissue to capture weak X-ray absorbing properties that are not detected through conventional X-ray imaging [13], thus showing promise as a novel diagnostic tool, similar to low dose CT. Briefly, due to the relatively high refractive index difference between lung tissue and air, XPC is a powerful tool for capturing details of the lung that may indicate the presence of COPD or other lung defects. As an example, a preliminary clinical study was able to successfully distinguish mild and severe emphysema in mice utilizing an analyzer-based XPC to map airway regions while retaining a sensitivity of 0.80 and a specificity of 0.89, showing its accuracy and potential application in pulmonary diagnostic processes that requires significant clinical development. Additionally, XPC can measure lung air volume capacity by capturing dynamic images of the lung, thus allowing observation of lung function changes at both the regional and global levels [14][15][16]. Briefly, there are five main categories of XPC imaging: (1) propagation-based imaging, (2) analyzer-based imaging, (3) interferometric methods using crystals, (4) grating interferometric, and (5) grating non-interferometric techniques. Each have their advantages and disadvantages, but all depend on X-ray detector resolution, image reconstruction algorithms, X-ray energy, and X-ray divergence for efficient result acquisition [17][18]. The specific advantages of different XPC imaging categories need to be developed in a clinical setting to ascertain the optimal method for capturing specific details and functions of the lung consistent with COPD. In spite of AI or algorithm-based analysis capabilities, resolution and radiation risk remain significant limitations for XPC’s clinical implementation. Moreover, this technique requires complex radiology equipment with limited scope in real-time, or routine bedside monitoring of COPD and respiratory disease progression for timely intervention.
2.2. Force and Impulse Oscillometry Measurements for Lung Function Analysis
As discussed above and previously [19], there is a significant unmet need for COPD diagnostic techniques that notably quantify real-time changes in lung function to detect COPD in its early stages for timely intervention. An example of such emerging COPD diagnostic is FOT, which noninvasively assesses lung function by measuring the lung’s response to applied pressure oscillations over normal breathing patterns and quantifies it as impedance, which is the resistance of a system airflow and is calculated from the pressure and airflow recorded throughout the measurement [20][21]. Similarly, IOS is a variation of FOT, where FOT transmits frequencies sequentially, while IOS transmits frequencies as an impulse that can be differentiated into varying frequencies, making the testing process faster while improving the signal-to-noise ratio [22][23], which is the most significant limitation of this technique. The impedance from these tests represents the responsiveness of the respiratory system, making FOT/IOS a significant prospective diagnostic tool for quantifying bronchial hyperresponsiveness, which is prevalent in patients with asthma and COPD. Although most of the research on the clinical application of FOT/IOS, mainly focuses on asthma and large airways, where FOT/IOS analysis shows its usefulness in detecting small airways for COPD identification depending on the frequency at which impedance is measured [24][25][26]. Additionally, applying machine learning to interpret FOT/IOS data after training the program with FOT/IOS data from smokers, COPD patients, and normal healthy subjects can further help improve the quantitative evaluation capability. As a proof of concept, this robust application of machine learning algorithms was found to be effective in detecting pulmonary changes with high degrees of specificity and sensitivity in various studies, showing its ability to detect subtle changes in lung function for early COPD detection [20][27]. Moreover, FOT/IOS does not require the subject to perform breathing maneuvers for measurements and only requires minimum compliance of the subject, thus making FOT/IOS useful over spirometry in a setting where patient compliance is limited, such as young children, subjects with chronic illnesses, or the elderly [28][29].
However, FOT has certain limitations as well, one example being that measurements can be affected by extra-thoracic upper airway artefacts that may distort the results when identifying small airway changes and COPD [30], thus preventing the accurate quantification of lung function and structural changes. Additionally, FOT cannot classify the causes of hyperresponsiveness, as other pulmonary deficiencies, such as asthma, may be responsible for functional change, necessitating other diagnostic tools or concurrent use of bronchodilators to confirm or validate the presence of COPD [31]. FOT/IOS is also limited in its ability to quantify regional lung function changes, as it only gauges the global impedance and hyperresponsiveness of the lung, rather than specific regions. In addition, one limitation specific to IOS is that the impulse pressure may be too intense as compared to the sequential waves of the FOT, making the patient uncomfortable [22].
2.3. Photoacoustic and Ultrasound Tomography as Emerging Lung Imaging Modalities
Another emerging diagnostic tool for COPD diagnosis is PAT, which serves as an improved alternative to the conventional CT scans that utilize ionizing radiation. Alternatively, low dose CT can reduce the risk of radiation, but it comes at the cost of decreased resolution. However, PAT uses signals from optical absorption to generate high-resolution images by exciting endogenous chromophores or exogenous contrast agents with laser beams, causing them to absorb the optical energy and increase in temperature, thus resulting in tissue expansion and, consequently, generating an ultrasound signal. This signal is then reconstructed using an algorithm to create an image that captures details of the airway and damage to lung tissue [32][33][34][35] that can then be analyzed for detection of COPD or other pulmonary disease [36]. This technique allows for improved spatial resolution, which is often limited in other optical imaging modalities due to light diffusion. Additionally, PAT does not contain optical contrasts or interfering speckle artefacts that are present in other ultrasonic imaging modalities. Moreover, PAT uses nonionizing radiation, making it a healthier alternative to modalities that utilize harmful ionizing radiation [32][37]. Nevertheless, PAT’s imaging capabilities are limited, as its imaging depth is dependent on the limit of attenuation caused by tissue [38]. PAT is further limited by its extended imaging times, which are restricted by the pulse repetition rate of the laser beams in optical excitation [32][39]. Additionally, like classical imaging modalities, PAT infers functional changes based on structural defects in the airway.
UCT is another emerging tomographic imaging modality that generates images by transmitting ultrasound waves into the tissue, which distort the waves before they are recorded by ultrasound transducers. Because UCT uses ultrasound waves instead of radiation or magnets, it can record properties of the sample that other imaging modalities are incapable of measuring, such as the attenuation of sound waves [40][41]. Additionally, UCT does not expose the subject to harmful ionizing radiation, making it a much healthier imaging modality than traditional radiation utilizing modalities. Despite its potential, UCT has not been used widely in pulmonary imaging, and its prospective application into the diagnostic field requires significant development of robust analysis tools to avoid high signal to noise ratios and to improve the quality of resolution. Overall, its soft-tissue imaging properties and innocuous imaging techniques show potential, yet are significantly limited, in capability for future-routine use lung disease diagnostics, as similar to current SOC diagnostic PFT, current prototypes cannot quantify regional or local function changes for capturing early or subtle lung function changes.
2.4. Electrical Impedance Tomography as Novel Diagnostics for Regional Lung Function Analysis
EIT is a promising emerging diagnostic tool that can quantify regional changes in the lung function with basic assessment and quantification of the changes in structure of the lung by non-invasively generating cross-sectional images through alternating low-dose current injections at a specific frequency via surface electrodes while measuring the changes in conductivity. This technology takes advantage of the fact that regions of muscle and blood have lower impedance than areas of fat, bone, air, and lung tissue due to free ion content, thus allowing for a ring of electrodes, generally 16 or 32, placed around the 4th and 5th intercostal space to measure impedance differences in regions of the lung, spanning all lobes. Moreover, conductivity changes in V and Q can then be reconstructed by an algorithm utilizing impedance measurements to produce images that can be used to evaluate COPD and quantify changes in lung function through comprehensive assessment of the V/Q maps. This data can be analyzed using novel quantification software(s), algorithms, and AI tools to identify specific changes in air or blood flow.
One method in which EIT has been used to detect COPD is by calculating the global heterogeneity Index (HI) [42][43] that is derived from the EIT V/Q or airflow (FEV1/FVC) heat maps collected during tidal breathing, allowing quantification of both ventilation heterogeneity and specific assessment of lung function. Briefly, comparison of HI of COPD and non-COPD groups revealed that the non-COPD group consistently had a lower global HI than the COPD group, demonstrating that EIT is capable of both distinguishing and identifying COPD. Furthermore, ventilation heterogeneity can be used to monitor COPD by utilizing inspiratory peaks and expiratory troughs from EIT measurements to plot heating and cooling maps of expiratory time (tE), phase shift (PHASE), and amplitude of impedance signal (AMP), in which the heat intensity maps can be utilized to demonstrate their corresponding HIs. Assessing this metric’s efficacy in COPD recognition showed that those with COPD had an overall increased ventilation heterogeneity and coefficient of variation [42], thus showing this tool’s capability in observing changes in lung function over a period of time to quantify pulmonary diseases progression. Furthermore, reconstruction of EIT data is used to calculate regional or local FEV1/FVC ratios by measuring impedance values during different time points in an inspiration/expiration maneuver, further showing its potential in providing standard output measures with regional assessment capability [44]. It is noteworthy that EIT is a safer and more robust alternative to traditional SOC modalities (as shown in Figure 2) for assessing regional lung function changes, as it does not expose the patient to harmful radiation or toxic chemicals or contrast agents, thus allowing continuous real time assessment of regional lung function for monitoring disease progression at the bedside or at POC.
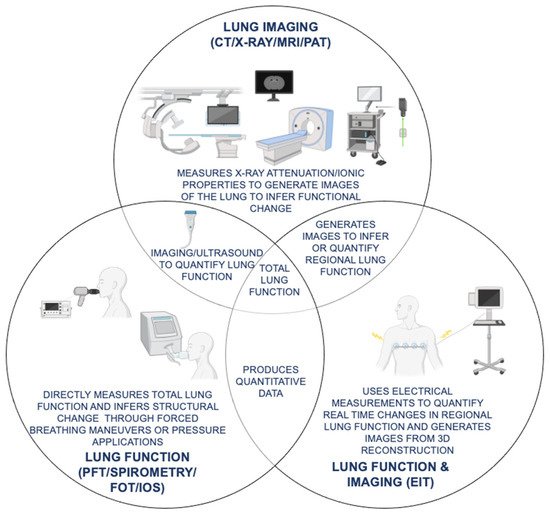
Figure 2. Comparison of current lung imaging and lung function tests with emerging oscillation and tomography techniques for COPD (chronic obstructive pulmonary disease) diagnosis and monitoring. Functional similarities and differences in SOC (standard of care) lung imaging (CT (computed tomography), X-ray, MRI (magnetic resonance imaging)) and lung function (PFT (pulmonary function test), spirometry, FOT (forced oscillation technique), IOS (impulse oscillometry system)) tests as compared to emerging novel modalities with both lung function and imaging capabilities (EIT (electrical impedance tomography), UCT (ultrasound computed tomography), PAT (photoacoustic tomography)) for COPD diagnosis and monitoring are shown.
In initial prototypes, EIT’s application as a diagnostic and monitoring tool was limited by its relatively low spatial resolution, as reconstructed images are not as detailed as those generated in traditional imaging modalities. However, EIT retains a high temporal resolution and quantitative assessment capabilities, making it a useful medium for monitoring regional changes [45] as compared to FOT/IOS, PAT, UCT, XPC, etc. Additionally, impedance measurements are very sensitive, capable of capturing subtle or narrow changes [46], necessitating the need for automation to minimize run-to-run variability. Moreover, currently available clinical EIT prototypes only measure cross-sections and, therefore, do not reflect conductivity measurements of regions of the lung in the z-axis. However, this limitation can be addressed easily by utilizing electrode devices designed for 3D imaging to reconstruct corresponding cross-sections of the lung and measuring it relative to time, allowing quantitative assessment of regional lung function changes.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10245811
References
- Halpin, D.M.G.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36.
- Hikichi, M.; Mizumura, K.; Maruoka, S.; Gon, Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J. Thorac. Dis. 2019, 11, S2129–S2140.
- Berend, N. Contribution of air pollution to COPD and small airway dysfunction. Respirology 2016, 21, 237–244.
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 307, L205–L218.
- Forey, B.A.; Thornton, A.J.; Lee, P.N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm. Med. 2011, 11, 36.
- Davis, R.M.; Novotny, T.E. The epidemiology of cigarette smoking and its impact on chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1989, 140, S82–S84.
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Kulmala, M.; Tiittanen, P.; Pekkanen, J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax 2008, 63, 635–641.
- DeVries, R.; Kriebel, D.; Sama, S. Outdoor Air Pollution and COPD-Related Emergency Department Visits, Hospital Admissions, and Mortality: A Meta-Analysis. COPD 2017, 14, 113–121.
- Ko, F.W.; Hui, D.S. Air pollution and chronic obstructive pulmonary disease. Respirology 2012, 17, 395–401.
- McGeachie, M.J. Childhood asthma is a risk factor for the development of chronic obstructive pulmonary disease. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 104–109.
- Brode, S.K.; Ling, S.C.; Chapman, K.R. Alpha-1 antitrypsin deficiency: A commonly overlooked cause of lung disease. CMAJ 2012, 184, 1365–1371.
- Silverman, E.K. Genetics of COPD. Annu. Rev. Physiol. 2020, 82, 413–431.
- Croton, L.C.P.; Morgan, K.S.; Paganin, D.M.; Kerr, L.T.; Wallace, M.J.; Crossley, K.J.; Miller, S.L.; Yagi, N.; Uesugi, K.; Hooper, S.B.; et al. In situ phase contrast X-ray brain CT. Sci. Rep. 2018, 8, 11412.
- Kitchen, M.J.; Buckley, G.A.; Kerr, L.T.; Lee, K.L.; Uesugi, K.; Yagi, N.; Hooper, S.B. Emphysema quantified: Mapping regional airway dimensions using 2D phase contrast X-ray imaging. Biomed. Opt. Express 2020, 11, 4176–4190.
- Kitchen, M.J.; Lewis, R.A.; Morgan, M.J.; Wallace, M.J.; Siew, M.L.; Siu, K.K.; Habib, A.; Fouras, A.; Yagi, N.; Uesugi, K.; et al. Dynamic measures of regional lung air volume using phase contrast x-ray imaging. Phys. Med. Biol. 2008, 53, 6065–6077.
- Lewis, R.A.; Yagi, N.; Kitchen, M.J.; Morgan, M.J.; Paganin, D.; Siu, K.K.; Pavlov, K.; Williams, I.; Uesugi, K.; Wallace, M.J.; et al. Dynamic imaging of the lungs using x-ray phase contrast. Phys. Med. Biol. 2005, 50, 5031–5040.
- Bravin, A.; Coan, P.; Suortti, P. X-ray phase-contrast imaging: From pre-clinical applications towards clinics. Phys. Med. Biol. 2013, 58, R1–R35.
- Zhou, W.; Majidi, K.; Brankov, J.G. Analyzer-based phase-contrast imaging system using a micro focus X-ray source. Rev. Sci. Instrum. 2014, 85, 085114.
- Vij, N. Prognosis-Based Early Intervention Strategies to Resolve Exacerbation and Progressive Lung Function Decline in Cystic Fibrosis. J. Pers. Med. 2021, 11, 96.
- Ribeiro, C.O.; Faria, A.C.D.; Lopes, A.J.; de Melo, P.L. Forced oscillation technique for early detection of the effects of smoking and COPD: Contribution of fractional-order modeling. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3281–3295.
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farré, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041.
- Desiraju, K.; Agrawal, A. Impulse oscillometry: The state-of-art for lung function testing. Lung India 2016, 33, 410–416.
- Bickel, S.; Popler, J.; Lesnick, B.; Eid, N. Impulse oscillometry: Interpretation and practical applications. Chest 2014, 146, 841–847.
- Bhattarai, P.; Myers, S.; Chia, C.; Weber, H.C.; Young, S.; Williams, A.D.; Sohal, S.S. Clinical Application of Forced Oscillation Technique (FOT) in Early Detection of Airway Changes in Smokers. J. Clin. Med. 2020, 9, 2778.
- Li, L.Y.; Yan, T.S.; Yang, J.; Li, Y.Q.; Fu, L.X.; Lan, L.; Liang, B.M.; Wang, M.Y.; Luo, F.M. Impulse oscillometry for detection of small airway dysfunction in subjects with chronic respiratory symptoms and preserved pulmonary function. Respir. Res. 2021, 22, 68.
- Faria, A.C.; Lopes, A.J.; Jansen, J.M.; Melo, P.L. Evaluating the forced oscillation technique in the detection of early smoking-induced respiratory changes. Biomed. Eng. Online 2009, 8, 22.
- Amaral, J.L.; Lopes, A.J.; Faria, A.C.; Melo, P.L. Machine learning algorithms and forced oscillation measurements to categorise the airway obstruction severity in chronic obstructive pulmonary disease. Comput. Methods Programs Biomed. 2015, 118, 186–197.
- Malmberg, L.P.; Mieskonen, S.; Pelkonen, A.; Kari, A.; Sovijärvi, A.R.; Turpeinen, M. Lung function measured by the oscillometric method in prematurely born children with chronic lung disease. Eur. Respir. J. 2000, 16, 598–603.
- Kalhoff, H.; Breidenbach, R.; Smith, H.J.; Marek, W. Impulse oscillometry in preschool children and association with body mass index. Respirology 2011, 16, 174–179.
- Uchida, A.; Ito, S.; Suki, B.; Matsubara, H.; Hasegawa, Y. Influence of cheek support on respiratory impedance measured by forced oscillation technique. Springerplus 2013, 2, 342.
- Kim, C.W.; Kim, J.S.; Park, J.W.; Hong, C.S. Clinical applications of forced oscillation techniques (FOT) in patients with bronchial asthma. Korean J. Intern. Med. 2001, 16, 80–86.
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22.
- Hou, R.; Le, T.; Murgu, S.D.; Chen, Z.; Brenner, M. Recent advances in optical coherence tomography for the diagnoses of lung disorders. Expert Rev. Respir. Med. 2011, 5, 711–724.
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631.
- Moore, C.; Jokerst, J.V. Strategies for Image-Guided Therapy, Surgery, and Drug Delivery Using Photoacoustic Imaging. Theranostics 2019, 9, 1550–1571.
- Zhao, Y.; Liu, W.; Tian, Y.; Yang, Z.; Wang, X.; Zhang, Y.; Tang, Y.; Zhao, S.; Wang, C.; Liu, Y.; et al. Anti-EGFR Peptide-Conjugated Triangular Gold Nanoplates for Computed Tomography/Photoacoustic Imaging-Guided Photothermal Therapy of Non-Small Cell Lung Cancer. ACS Appl. Mater. Interfaces 2018, 10, 16992–17003.
- Kim, J.; Lee, D.; Jung, U.; Kim, C. Photoacoustic imaging platforms for multimodal imaging. Ultrasonography 2015, 34, 88–97.
- Burgholzer, P.; Bauer-Marschallinger, J.; Reitinger, B.; Berer, T. Resolution Limits in Photoacoustic Imaging Caused by Acoustic Attenuation. J. Imaging 2019, 5, 13.
- Upputuri, P.K.; Pramanik, M. Fast photoacoustic imaging systems using pulsed laser diodes: A review. Biomed. Eng. Lett. 2018, 8, 167–181.
- Jago, J.R.; Whittingham, T.A. Experimental studies in transmission ultrasound computed tomography. Phys. Med. Biol. 1991, 36, 1515–1527.
- Liu, C.; Xue, C.; Zhang, B.; Zhang, G.; He, C. The Application of an Ultrasound Tomography Algorithm in a Novel Ring 3D Ultrasound Imaging System. Sensors 2018, 18, 1332.
- Milne, S.; Huvanandana, J.; Nguyen, C.; Duncan, J.M.; Chapman, D.G.; Tonga, K.O.; Zimmermann, S.C.; Slattery, A.; King, G.G.; Thamrin, C. Time-based pulmonary features from electrical impedance tomography demonstrate ventilation heterogeneity in chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 127, 1441–1452.
- Trenk, F.; Mendes, L.; Carvalho, P.; Paiva, R.P.; Henriques, J.; Maglaveras, N.; Chouvarda, I.; Tsara, V.; Teixeira, C.A. Evaluation of lung ventilation distribution in chronic obstructive pulmonary disease patients using the global inhomogeneity index. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; 2016, pp. 5286–5289.
- Vogt, B.; Deuß, K.; Hennig, V.; Zhao, Z.; Lautenschläger, I.; Weiler, N.; Frerichs, I. Regional lung function in nonsmokers and asymptomatic current and former smokers. ERJ Open Res. 2019, 5, 240.
- Schullcke, B.; Gong, B.; Krueger-Ziolek, S.; Soleimani, M.; Mueller-Lisse, U.; Moeller, K. Structural-functional lung imaging using a combined CT-EIT and a Discrete Cosine Transformation reconstruction method. Sci. Rep. 2016, 6, 25951.
- Braun, F.; Proença, M.; Lemay, M.; Bertschi, M.; Adler, A.; Thiran, J.P.; Solà, J. Limitations and challenges of EIT-based monitoring of stroke volume and pulmonary artery pressure. Physiol. Meas. 2018, 39, 014003.
This entry is offline, you can click here to edit this entry!
