Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Leptin, an adipokine regulating body fat mass, represents a key molecule in obesity, able to modulate immune responses and foster chronic inflammatory response in peripheral tissues.
- leptin
- obesity
- immune regulation
- Physiology
1. Physiological Role of Leptin and Its Relationship with Obesity
Leptin is a hormone secreted by white adipocytes [1][2]. Through the blood-brain barrier, such a hormone reaches the hypothalamus to decrease food intake and to increase metabolism [1]. Leptin receptors, encoded by the LEPR gene [3][4], are expressed by hypothalamic satiety centres and are widely disseminated throughout the body—this occurrence reflects the pleiotropic nature of leptin that is involved in the control of many physiologic processes [5]. Ob-Rb, the ‘long’ isoform of the receptor, is predominantly expressed in the hypothalamus [6][7][8], while the short isoforms (Ob-Ra, Ob-Rc, Ob-Rd, and Ob-Rf) are expressed in the peripheral tissues [9][10]. Leptin receptor (LEPR) needs the activation of receptor associated kinases of Janus family (JAKs), which in turn induce downstream signalling involving different members of signal transducers and activators of transcription (STAT) family [11]. Leptin receptors activate a complex neural circuit involving anorexigenic (i.e., appetite-diminishing) and orexigenic (i.e., appetite-stimulating) neuropeptides to control food intake.
Moreover, leptin also stimulates the sympathetic nervous system inducing an increase in plasma norepinephrine and epinephrine concentrations via the ventromedial hypothalamus [12].
In addition to its pivotal role in the regulation of energy metabolism [13], leptin possesses other important physiological activities as the control of neuroendocrine and immune functions, and haematopoiesis [14][15]. The strict association between obesity and hematopoietic disruption evidenced the role of leptin on bone organization. The direct role for leptin in haematopoiesis has been demonstrated by the presence of Ob-R on bone marrow CD34+ cells as well as on lympho-haematopoietic and megakaryocytic cell lines [16][17]. Recently, Claycombe et al. [18] demonstrated that myelopoiesis recover after treatment with leptin in obese mice (ob/ob). Aberrant leptin levels in patients with haematological malignancies have been described, suggesting that leptin signalling is involved in the progression of haematological malignancies and could represent a useful prognostic value [19].
Relationship between leptin and obesity could be considered as a part of metabolic syndrome (MS), the pathological condition comprising of also dyslipidaemia, hyperglycaemia, and high blood pressure. It is noteworthy that obesity is related to the leptin receptor resistance mechanisms [20], including several aspects such as: (i) Interruption of leptin signalling in hypothalamic and other central nervous system (CNS) neurons; (ii) alteration of leptin transport across blood-brain barrier; (iii) hypothalamic inflammation, autophagy, and endoplasmic reticulum stress [21][22]. The development of leptin resistance and of hyperleptinemia have been widely demonstrated in humans and in domestic animals [23].
In the course of obesity and hyperleptinemia condition, an accumulation of epicardial adipose tissue has been demonstrated [24], suggesting its involvement in cardiovascular system damage. Chronic inflammation and the accumulation of epicardial fat is strongly concomitant with coronary artery disease, independent of visceral adiposity [24]. Furthermore, high circulating levels of leptin appeared to induce significant impairment of the haemostatic balance in cardiovascular diseases [25].
Moreover, leptin has been associated to hypertension and congestive heart failure (HF) in humans, dogs, and cats [23][26]. In addition, leptin accelerates atherosclerosis spreading [27].
The role of leptin and adipokines on the cardiovascular system have been largely described to be dependent on two mechanisms involving the heart or the central nervous system [28][29][30]. Leptin acts by stimulating the migration and proliferation of vascular smooth muscle cells (VSMCs) [31]. Such hormones block the vasoconstrictor action of angiotensin II and inhibits the angiotensin II-induced increase in intracellular Ca2+ in VSMCs through Ob-Rb [32]. Leptin shows angiogenetic effects dependent on both proliferation and migration of vascular smooth muscle cells by promoting the upregulation of vascular endothelial growth factor (VEGF) expression [33] and the cytoskeleton reorganization [34].
Acute pancreatitis is associated with high levels of leptin in serum and pancreas [35][36], suggesting the role for such a hormone as a marker for adipose tissue necrosis [37]. Intriguingly, the pancreas could secrete leptin and its protective role in pancreatitis has been described [38][39]. In agreement with this hypothesis, beneficial effects of leptin on acute pancreatitis have been evidenced in ischemia/reperfusion [39][40].
2. Role of Leptin in the Relationship between Obesity and Immune-Modulation.
An interesting scenario on obesity is that immune response greedily needs “energy” to be implemented. In a pathophysiological perspective, this energy can be in excess or in deficit. In this regard, food opulence is frequently associated with autoimmune diseases [41][42][43], while hyponutrition induces susceptibility to infectious diseases [44][45][46][47]. Therefore, an excess of nutrients could drive the immune system towards self-reactivity, while a defect can determine insufficient anti-infectious immune responses. In this regard, the relationship between obesity and immune modulation appears of great relevance in both human and veterinary medicine [41][42][43][48][49][50][51][52][53].
In human and animal obesity, the secretion of leptin and other hormones from the adipose tissue appears to determine the dysregulation of the immune response [41][54][55] (Figure 1).
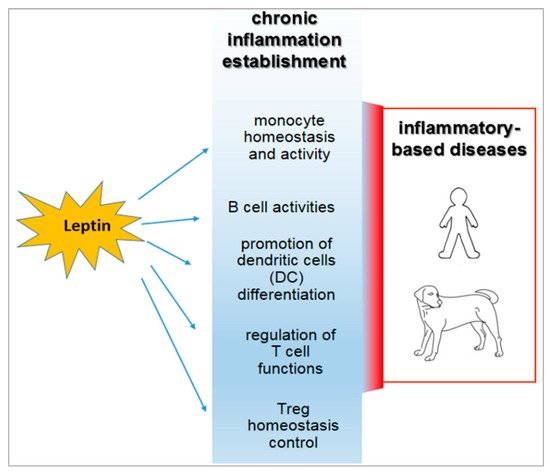
Figure 1. Inflammatory roles of leptin in the course of obesity and their relevance in both human and veterinary medicine.
Moreover, leptin and its receptors are integral components of a complex physiological system evolved to regulate fuel stores and energy balance at an optimum level in mammals [56].
Leptin has structural similarities with the alpha-helix family of cytokines and its receptor (ObR) belongs to the superfamily of class I cytokine receptors [57]. Leptin receptors are expressed by immune system cells [58][59][60], and leptin possesses modulatory effects on both innate and adaptive immunity [61][62] (Figure 2). Such a hormone is currently considered a pro-inflammatory adipokine [41][63][64]. In this regard, leptin acts as an acute phase inflammatory cytokine like interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF)-α [14] and is necessary for phagocytosis of bacteria by polymorph nuclear cells [65].
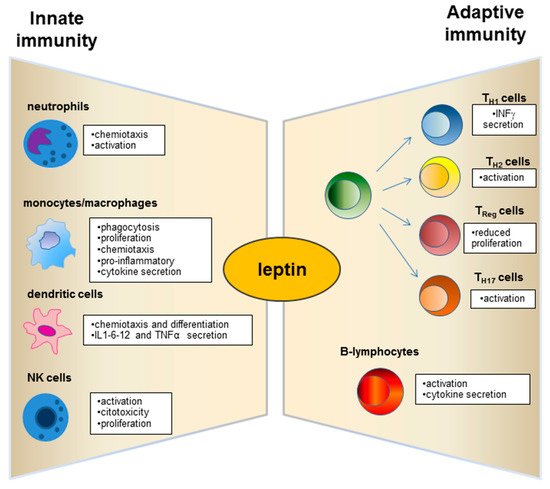
Figure 2. Physiological role of leptin on innate and adaptive immunity.
Neutrophils express the short form of the leptin receptor [66] that can stimulate the expression of CD11b and prevent apoptosis.
Dendritic cells (DC), a specialized cell population for antigen uptake in body tissues, express leptin receptors (Ob-R) on their surface [67]. Leptin acts on these cells, favouring their differentiation, maturation, recruitment, and survival [67][68] and modulating the signalling pathways involved in these biological processes as observed in db/db mice lacking leptin receptors (Ob-R) [68]. Furthermore, an important role of leptin is exercised by the activation and recruitment of the DC (Figure 2).
Deficits of leptin receptors in Natural Killer (NK) cells correlate with decreased NK number and functions [69][70].
The role of leptin in adaptive immunity has been largely demonstrated from early studies on db/db mice that showed high level of thymocyte apoptosis [72].
A great research interest has moved to explore the leptin role on the T and B cell population (Figure 2). Leptin acts with several mechanisms on T lymphocytes and induces the expression of the long isoform of LEPR in CD4+ T cells [73]. Such adipokine promotes activation and proliferation of T lymphocytes and enhances their cytokine production [74][75]. In addition, the leptin supplementation to a mixed lymphocyte reaction has been observed to induce a proliferation of CD4+ T cells [75].
Leptin regulates the adaptive immunity, also influencing activities of T Helper (Th) 1 and 2 lymphocytes [41][63][44][76]. In particular, the hormone stimulates the Th1 production of cytokines such as IL-2, interferon (IFN)-γ, TNF-α, and IL-18, and drives the differentiation of the Th17 cells mainly involved in chronic inflammation establishment [77][78].
In addition, leptin influences B-cell activities, regulating and promoting cell cycle by Bcl-2 and cyclin D activation [79].
It is of note that leptin acts on the homeostasis of a specific CD4+CD25highFoxp3+ T immune regulatory cell population, usually referred to as Treg [41][80][81][82][83][84]. Such cells avoid the auto reactivity of the immune system against the “self” molecular components that belong to the individual itself [41][80][81][82]. Human Treg cells display heterogeneous gene expression, phenotype, and suppressive functions [85]. This occurrence strongly correlates with the different splicing variants of the transcriptional factor FoxP3 [86]—the full-length isoform (FoxP3fl), which contains the sequences involved in the interaction with retinoic acid-related orphan receptors α and γt (RORα and RORγt), is associated with Treg function in humans [87]. In contrast, the expression of the isoform lacking exon 2 (FoxP3Δ2) correlates with dysfunction of Treg cells, since it appears to be unable to interact with RORα and RORγt [88]. FoxP3Δ2 expression has been correlated with multiple sclerosis in humans [89]. Expression of the different FOXP3 isoforms is conditioned by metabolic aspects [90] and by the exposure of Treg to the pro-inflammatory micro-environment [91]. No data over this potential functional dichotomy are available from canine or feline models.
Nutrient availability is essential for the maintenance of tissue homeostasis. In this context, the intracellular “sensor” of nutrients [92] is represented by the mammalian target of rapamycin, the mTOR molecule [93]. This serine–threonine kinase “senses” the extracellular bioavailability of amino acids, glucose, growth factors, and hormones [81][92][93][94], promotes cell metabolism and growth when the conditions are favourable; or catabolic processes when conditions are not favourable. In this context, mTOR is strongly correlated with Treg homeostasis and functions [95]. High levels of leptin correlate with a reduced number and with decreased functions of Treg cells in human autoimmune diseases [41][42]. The relationship between metabolism and cell plasticity is of great relevance, particularly for the homeostasis of immune system cells that are highly “sensitive” to bioavailable nutrients [96][97][98][99].
Adiposity has been associated with increased concentrations of leptin and other proinflammatory adipokines, cytokines, and acute-phase proteins [100]. The role of adiponectin in dogs still appears controversial and few data are available in the veterinary literature on the possible impact of obesity on the immune response. The effects of weight loss on canine adipokines and cytokines have been reported [101][102][103][104][105]. Several studies showed that plasma leptin concentrations correlate with body fat content in experimentally induced obese beagles [106][107]. In this regard, Sagawa et al. highlighted that the positive relationship between plasma leptin concentration and body fat content in dogs is similar to correlations reported for humans and rodents [106]. Ishioka et al. [108] showed that plasma leptin represents an index of adiposity in dogs regardless of their age, gender, and breed variations. It is well known that plasma leptin concentrations increase with weight gain and decrease with weight loss in dogs. In this regard, Jeusette et al. [109] described a decrease in ghrelin and an increase in leptin and insulin concentrations in obese beagle dogs. The same authors [109] suggested that ghrelin and leptin could play a role in dogs in the adaptation to a positive or negative energy balance, as observed in humans. Proinflammatory state directly influences glucose metabolism, resulting in decreased insulin sensitivity [100]. In fact, high-plasma leptin concentrations have been correlated to insulin resistance in humans [110] and in insulin-resistant dogs [109]. Serum leptin concentrations correlated with percentage of body fat and decreased with weight loss, whereas the involvement of other inflammatory markers in canine obesity and weight loss is still less understood. Induction of canine obesity has been shown to increase concentrations of TNF-α [111] which decreases after a weight loss program in obese dogs [101]. However, acute phase proteins appeared to be unaltered after the weight loss program [103], while the production of C-reactive protein decreased in obese dogs [101][103][104][105][112].
Van de Velde et al. [113] investigated the effect of a short-term increase in body weight on immunological variables in adult healthy beagle dogs in which weight gain and increased body condition score (BCS) were accompanied by a significantly higher leptin concentration. Subsequently, the same authors [114] described that T-cell proliferation is affected after weight gain in Beagle dogs.
Recently, concentrations of IL-6 and monocyte chemoattractant protein 1, but not IL-8, were found to be increased in overweight dogs [115], whereas other authors described decreasing concentrations of IL-8 and other interleukins with weight loss in dogs [105]. Piantedosi et al. [116] revealed no significant differences in serum TNF-α and IL-6 concentrations between obese and normal weight dogs.
Increased inflammatory response has been correlated with clinical exacerbation, and the immunotherapeutic role of Tregs appears to be relevant in leishmaniosis [117].
Tregs function, macrophage activation, and the proinflammatory state appear to be involved in the pathogenesis of canine leishmaniasis. Naturally L. infantum infected dogs expressed alteration in leptin gene transcription and low levels of circulating Treg [118]. In the same model, ineffective immune response to parasites appeared to be associated with high Treg levels [119]. Di Loria et al. [120] showed an increase in leptin mRNA expression in dogs naturally infected by L. infantum.
This entry is adapted from the peer-reviewed paper 10.3390/ijms20102392
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432.
- Havel, P.J. Role of adipose tissue in body-weight regulation: Mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000, 59, 359–371.
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635.
- Armağan, C.; Yılmaz, C.; Koç, A.; Abac, A.; Ülgenalp, A.; Böber, E.; Erçal, D.; Demir, K. A toddler with a novel LEPR mutation. Hormones 2019.
- Wasim, M.; Awan, F.R.; Najam, S.S.; Khan, A.R.; Khan, H.N. Role of Leptin Deficiency, Inefficiency, and Leptin Receptors in Obesity. Biochem. Genet. 2016, 54, 565–572.
- Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 1997, 272, 6093–6096.
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15, 50–54.
- Fei, H.; Okano, H.J.; Li, C.; Lee, G.H.; Zhao, C.; Darnell, R.; Friedman, J.M. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 7001–7005.
- Yamashita, T.; Murakami, T.; Otani, S.; Kuwajima, M.; Shima, K. Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem. Biophys. Res. Commun. 1998, 246, 752.
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1407–1433.
- Frühbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006, 393, 7–20.
- Satoh, N.; Ogawa, Y.; Katsuura, G.; Numata, Y.; Tsuji, T.; Hayase, M.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Yoshimasa, Y.; et al. Sympathetic activation of leptin via the ventromedial hypothalamus: Leptin-induced increase in catecholamine secretion. Diabetes 1999, 48, 1787–1793.
- Dalamaga, M.; Chou, S.H.; Shields, K.; Papageorgiou, P.; Polyzos, S.A.; Mantzoros, C.S. Leptin at the intersection of neuroendocrinology and metabolism: Current evidence and therapeutic perspectives. Cell Metabol. 2013, 18, 29–42.
- Fantuzzi, G.; Faggioni, R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J. Leukoc. Biol. 2000, 68, 437–446.
- Pérez-Pérez, A.; Vilariño-García, T.; Fernández-Riejos, P.; Martín-González, J.; Segura-Egea, J.J.; Sánchez-Margalet, V. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017, 35, 71–84.
- Gainsford, T.; Willson, T.A.; Metcalf, D.; Handman, E.; McFarlane, C.; Nq, A.; Nicola, N.A.; Alexander, W.S.; Hilton, D.J. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 14564–14568.
- Bennett, B.D.; Solar, G.P.; Yuan, J.Q.; Mathias, J.; Thomas, G.R.; Matthews, W. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 1996, 6, 1170–1180.
- Claycombe, K.; King, L.E.; Fraker, P.J. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc. Natl. Acad. Sci. USA 2008, 105, 2017–2021.
- Han, T.J.; Wang, X. Leptin and its receptor in hematologic malignancies. Int. J. Clin. Exp. Med. 2015, 8, 19840–19849.
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252.
- Myers, M.G., Jr.; Heymsfield, S.B.; Haft, C.; Kahn, B.B.; Laughlin, M.; Leibel, R.L.; Tschöp, M.H.; Yanovski, J.A. Challenges and opportunities of defining clinical leptin resistance. Cell Metabol. 2012, 15, 150–156.
- Jung, C.H.; Kim, M.S. Molecular mechanisms of central leptin resistance in obesity. Arch. Pharm. Res. 2013, 36, 201–207.
- Zoran, D.L. Obesity in dogs and cats: A metabolic and endocrine disorder. Vet. Clin. North Am. Small Anim. Pract. 2010, 40, 221–239.
- Packer, M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372.
- Russo, I. The prothrombotic tendency in metabolic syndrome: Focus on the potential mechanisms involved in impaired haemostasis and fibrinolytic balance. Scientifica 2012, 2012, 525374.
- Barbosa-Ferreira, J.M.; Fernandes, F.; Dabarian, A.; Mady, C. Leptin in heart failure. Expert. Opin. Med. Diagn. 2013, 7, 113–117.
- Chiba, T.; Shinozaki, S.; Nakazawa, T.; Kawakami, A.; Ai, M.; Kaneko, E.; Kitagawa, M.; Kondo, K.; Chait, A.; Shimokado, K. Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis 2008, 196, 68–75.
- Lopaschuk, G.D.; Folmes, C.D.; Stanley, W.C. Cardiac energy metabolism in obesity. Circ. Res. 2007, 101, 335–347.
- Tune, J.D.; Considine, R.V. Effects of leptin on cardiovascular physiology. J. Am. Soc. Hypertens. 2007, 1, 231–241.
- Balasubramanian, P.; Hall, D.; Subramanian, M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. Geroscience 2019, 41, 13–24.
- Oda, A.; Taniguchi, T.; Yokoyama, M. Leptin stimulates rat aortic smooth muscle cell proliferation and migration. Kobe J. Med. Sci. 2001, 47, 141–150.
- Fortuño, A.; Rodríguez, A.; Gómez-Ambrosi, J.; Muñiz, P.; Salvador, J.; Díez, J.; Frühbeck, G. Leptin inhibits angiotensin II-induced intracellular calcium increase and vasoconstriction in the rat aorta. Endocrinology 2002, 143, 3555–3560.
- Suganami, T.; Mukoyama, M.; Mori, K.; Yokoi, H.; Koshikawa, M.; Sawai, K.; Hidaka, S.; Ebihara, K.; Tanaka, T.; Sugawara, A.; et al. Prevention and reversal of renal injury by leptin in a new mouse model of diabetic nephropathy. FASEB J. 2005, 19, 127–129.
- Morales-Ruiz, M.; Fulton, D.; Sowa, G.; Languino, L.R.; Fujio, Y.; Walsh, K.; Sessa, W.C. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 2000, 86, 892–896.
- Konturek, P.C.; Jaworek, J.; Maniatoglou, A.; Bonior, J.; Meixner, H.; Konturek, S.J.; Hahn, E.G. Leptin modulates the inflammatory response in acute pancreatitis. Digestion 2002, 65, 149–160.
- Frossard, J.L.; Lescuyer, P.; Pastor, C.M. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J. Gastroenterol. 2009, 15, 5260–5265.
- Karpavicius, A.; Dambrauskas, Z.; Sileikis, A.; Vitkus, D.; Strupas, K. Value of adipokines in predicting the severity of acute pancreatitis: Comprehensive review. World J. Gastroenterol. 2012, 18, 6620–6627.
- Konturek, P.C.; Konturek, S.J.; Brzozowski, T.; Jaworek, J.; Hahn, E.G. Role of leptin in the stomach and the pancreas. J. Physiol. 2001, 95, 345–354.
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Jaworek, J.; Konturek, P.C.; Dembiński, M.; Bilskl, J.; Konturek, S.J. Influence of leptin administration on the course of acute ischemic pancreatitis. J. Physiol. Pharmacol. 2002, 53, 775–790.
- Gultekin, F.A.; Kerem, M.; Tatlicioglu, E.; Aricioglu, A.; Unsal, C.; Bukan, N. Leptin treatment ameliorates acute lung injury in rats with cerulein-induced acute pancreatitis. World J. Gastroenterol. 2007, 13, 2932–2938.
- La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 2017, 98, 51–58.
- Carbone, F.; La Rocca, C.; De Candia, P.; Procaccini, C.; Colamatteo, A.; Micillo, T.; De Rosa, V.; Matarese, G. Metabolic control of immune tolerance in health and autoimmunity. Semin. Immunol. 2016, 28, 491–504.
- Medina, G.; Vera-Lastra, O.; Peralta-Amaro, A.L.; Jiménez-Arellano, M.P.; Saavedra, M.A.; Cruz-Domínguez, M.P.; Jara, L.J. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol. Res. 2018, 133, 277–288.
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588.
- Farhadi, S.; Ovchinnikov, R.S. The relationship between nutrition and infectious diseases: A review. Biomed. Biotechnol. Res. J. 2018, 2, 168–172.
- Hennig, B.; Petriello, M.C.; Gamble, M.V.; Surh, Y.J.; Kresty, L.A.; Frank, N.; Rangkadilok, N.; Ruchirawat, M.; Suk, W.A. The role of nutrition in influencing mechanisms involved in environmentally mediated diseases. Rev. Environ. Health 2018, 33, 87–97.
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741.
- Naylor, C.; Petri, W.A., Jr. Leptin regulation of immune responses. Trends Mol. Med. 2016, 22, 88–98.
- Maldonado-Ruiz, R.; Fuentes-Mera, L.; Camacho, A. Central modulation of neuroinflammation by neuropeptides and energy-sensing hormones during obesity. Biomed. Res. Int. 2017, 2017, 7949582.
- Lourenço, E.V.; Liu, A.; Matarese, G.; La Cava, A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc. Natl. Acad. Sci. USA 2016, 113, 10637–10642.
- Palatucci, A.T.; Piantedosi, D.; Rubino, V.; Giovazzino, A.; Guccione, J.; Pernice, V.; Ruggiero, G.; Cortese, L.; Terrazzano, G. Circulating regulatory T cells (Treg), leptin and induction of proinflammatory activity in obese Labrador Retriever dogs. Vet. Immunol. Immunopathol. 2018, 202, 122–129.
- Kawauchi, I.M.; Jeremias, J.T.; Takeara, P.; de Souza, D.F.; Balieiro, J.C.C.; Pfrimer, K.; Brunetto, M.A.; Pontieri, C.F.F. Effect of dietary protein intake on the body composition and metabolic parameters of neutered dogs. J. Nutr. Sci. 2017, 6, e40.
- Kim, A.Y.; Kim, H.S.; Kang, J.H.; Yang, M.P. Serum adipokine concentrations in dogs with diabetes mellitus: A pilot study. J. Vet. Sci. 2015, 16, 333–340.
- Lam, Q.L.; Lu, L. Role of leptin in immunity. Cell. Mol. Immunol. 2007, 4, 1–13.
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640.
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770.
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271.
- Matarese, G.; La Cava, A. The intricate interface between immune system and metabolism. Trends Immunol. 2004, 25, 193–200.
- Chan, J.L.; Matarese, G.; Shetty, G.K.; Raciti, P.; Kelesidis, I.; Aufiero, D.; De Rosa, V.; Perna, F.; Fontana, S.; Mantzoros, C.S. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 8481–8486.
- Procaccini, C.; Jirillo, E.; Matarese, G. Leptin as an immunomodulator. Mol. Aspects Med. 2012, 33, 35–45.
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379.
- Bernotiene, E.; Palmer, G.; Gabay, C. The role of leptin in innate and adaptive immune responses. Arthritis. Res. Ther. 2006, 8, 217.
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109.
- Lago, F.; Gómez, R.; Gómez-Reino, J.J.; Dieguez, C.; Gualillo, O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 2009, 34, 500–510.
- Hsu, A.; Aronoff, D.M.; Phipps, J.; Goel, D.; Mancuso, P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin. Exp. Immunol. 2007, 150, 332–339.
- Zarkesh-Esfahani, H.; Pockley, A.G.; Wu, Z.; Hellewell, P.G.; Weetman, A.P.; Ross, R.J. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J. Immunol. 2004, 172, 1809–1814.
- Mattioli, B.; Straface, E.; Quaranta, M.G.; Giordani, L.; Viora, M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 2005, 174, 6820–6828.
- Lam, Q.L.K.; Liu, S.; Cao, X.; Lu, L. Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur. J. Immunol. 2006, 36, 3118–3130.
- Laue, T.; Wrann, C.D.; Hoffmann-Castendiek, B.; Pietsch, D.; Hübner, L.; Kielstein, H. Altered NK cell function in obese healthy humans. BMC Obes. 2015, 2, 1.
- Jahn, J.; Spielau, M.; Brandsch, C.; Stangl, G.I.; Delank, K.S.; Bähr, I.; Berreis, T.; Wrann, C.D.; Kielstein, H. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity 2015, 23, 2233–2241.
- Tian, Z.; Sun, R.; Wei, H.; Gao, B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: Leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 2002, 298, 297–302.
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 1999, 104, 1051–1059.
- Sánchez-Margalet, V.; Martín-Romero, C.; González-Yanes, C.; Goberna, R.; Rodríguez-Baño, J.; Muniain, M.A. Leptin receptor (Ob-R) expression is induced in peripheral blood mononuclear cells by in vitro activation and in vivo in HIV-infected patients. Clin. Exp. Immunol. 2002, 129, 119–124.
- Martín-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000, 199, 15–24.
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901.
- Batra, A.; Okur, B.; Glauben, R.; Erben, U.; Ihbe, J.; Stroh, T.; Fedke, I.; Chang, H.D.; Zeitz, M.; Siegmund, B. Leptin: A critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology 2010, 151, 56–62.
- Deng, J.; Liu, Y.; Yang, M.; Wang, S.; Zhang, M.; Wang, X.; Ko, K.H.; Hua, Z.; Sun, L.; Cao, X.; et al. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 2012, 64, 3564–3573.
- Reis, B.S.; Lee, K.; Fanok, M.H.; Mascaraque, C.; Amoury, M.; Cohn, L.B.; Rogoz, A.; Dallner, O.S.; Moraes-Vieira, P.M.; Domingos, A.I.; et al. Leptin receptor signaling in T cells is required for Th17 differentiation. J. Immunol. 2015, 194, 5253–5260.
- Lam, Q.L.K.; Wang, S.; Ko, O.K.H.; Kincade, P.W.; Lu, L. Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc. Natl Acad. Sci. USA 2010, 107, 13812–13817.
- Sakaguchi, S. The origin of FOXP3-expressing CD4+ regulatory T cells: Thymus or periphery. J. Clin. Investig. 2003, 112, 1310–1312.
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–245.
- Silva Morales, M.; Mueller, D. Anergy into T regulatory cells: An integration of metabolic cues and epigenetic changes at the Foxp3 conserved non-coding sequence 2. F1000Research 2018, 7, 1938.
- Kumar, P.; Bhattacharya, P.; Prabhakar, B.S. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J. Autoimmun. 2018, 95, 77–99.
- Matarese, G.; Procaccini, C.; De Rosa, V.; Horvath, T.L. La Cava, A. Regulatory T cells in obesity: The leptin connection. Trends Mol. Med. 2010, 16, 247–256.
- Lopes, J.E.; Torgerson, T.R.; Schubert, L.A.; Anover, S.D.; Ocheltree, E.L.; Ochs, H.D.; Ziegler, S.F. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J. Immunol. 2006, 177, 3133–3142.
- Ito, T.; Hanabuchi, S.; Wang, Y.H.; Park, W.R.; Arima, K.; Bover, L.; Qin, F.X.; Gilliet, M.; Liu, Y.J. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 2008, 28, 870–880.
- Du, J.; Huang, C.; Zhou, B.; Ziegler, S.F. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J. Immunol. 2008, 180, 4785–4792.
- Zhou, L.; Lopes, J.E.; Chong, M.M.; Ivanov, I.I.; Min, R.; Victora, G.D.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008, 453, 236–240.
- Sambucci, M.; Gargano, F.; De Rosa, V.; De Bardi, M.; Picozza, M.; Placido, R.; Ruggieri, S.; Capone, A.; Gasperini, C.; Matarese, G.; et al. FoxP3 isoforms and PD-1 expression by T regulatory cells in multiple sclerosis. Sci. Rep. 2018, 8, 3674.
- De Rosa, V.; Galgani, M.; Porcellini, A.; Colamatteo, A.; Santopaolo, M.; Zuchegna, C.; Romano, A.; De Simone, S.; Procaccini, C.; La Rocca, C.; et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015, 16, 1174–1184.
- Mailer, R.K.; Joly, A.L.; Liu, S.; Elias, S.; Tegner, J.; Andersson, J. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci. Rep. 2015, 5, 14674.
- McLeod, L.E.; Proud, C.G. ATP depletion increases phosphorylation of elongation factor eEF2 in adult cardiomyocytes independently of inhibition of mTOR signalling. FEBS Lett. 2002, 531, 448–452.
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell. Sci. 2009, 122, 3589–3594.
- Galgani, M.; Procaccini, C.; De Rosa, V.; Carbone, F.; Chieffi, P.; La Cava, A.; Matarese, G. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J. Immunol. 2010, 185, 7474–7479.
- Zeng, H.; Yang, K.; Cloer, C.; Neale, G.; Vogel, P.; Chi, H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 2013, 499, 485–490.
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35, 871–882.
- Donnelly, R.P.; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015, 68, 513–519.
- Blagih, J.; Coulombe, F.; Vincent, E.E.; Dupuy, F.; Galicia-Vázquez, G.; Yurchenko, E.; Raissi, T.C.; van der Windt, G.J.; Viollet, B.; Pearce, E.L.; et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 2015, 42, 41–54.
- Loftus, R.M.; Finlay, D.K. Immunometabolism: Cellular metabolism turns immune regulator. J. Biol. Chem. 2016, 291, 1–10.
- Antuna-Puente, B.; Feve, B.; Fellahi, S.; Bastard, J.P. Adipokines: The missing link between insulin resistance and obesity. Diabetes Metab. 2008, 34, 2–11.
- Pradhan, A. Obesity, metabolic syndrome, and type 2 diabetes: Inflammatory basis of glucose metabolic disorders. Nutr. Rev. 2007, 65, S152–S156.
- Mihalko, W.M.; Bergin, P.F.; Kelly, F.B.; Canale, S.T. Obesity, orthopaedics, and outcomes. J. Am. Acad. Orthop. Surg. 2014, 22, 683–690.
- Tvarijonaviciute, A.; Tecles, F.; Martinez-Subiela, S.; Ceron, J.J. Effect of weight loss on inflammatory biomarkers in obese dogs. Vet. J. 2012, 193, 570–572.
- Wakshlag, J.J.; Struble, A.M.; Levine, C.B.; Bushey, J.J.; Laflamme, D.P.; Long, G.M. The effects of weight loss on adipokines and markers of inflammation in dogs. Br. J. Nutr. 2011, 106, S11–S14.
- Bastien, B.C.; Patil, A.; Satyaraj, E. The impact of weight loss on circulating cytokines in Beagle dogs. Vet. Immunol. Immunopathol. 2015, 163, 174–182.
- Sagawa, M.M.; Nakadomo, F.; Honjoh, T.; Ishioka, K.; Saito, M. Correlation between plasma leptin concentration and body fat content in dogs. Am. J. Vet. Res. 2002, 63, 7–10.
- Ishioka, K.; Soliman, M.M.; Sagawa, M.; Nakadomo, F.; Shibata, H.; Honjoh, T.; Hashimoto, A.; Kitamura, H.; Kimura, K.; Saito, M. Experimental and clinical studies on plasma leptin in obese dogs. J. Vet. Med. Sci. 2002, 64, 349–353.
- Ishioka, K.; Hosoya, K.; Kitagawa, H.; Shibata, H.; Honjoh, T.; Kimura, K.; Saito, M. Plasma leptin concentration in dogs: Effects of body condition score, age, gender and breeds. Res. Vet. Sci. 2007, 82, 11–15.
- Jeusette, I.C.; Detilleux, J.; Shibata, H.; Saito, M.; Honjoh, T.; Delobel, A.; Istasse, L.; Diez, M. Effects of chronic obesity and weight loss on plasma ghrelin and leptin concentrations in dogs. Res. Vet. Sci. 2005, 79, 169–175.
- Segal, K.R.; Landt, M.; Klein, S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 1996, 45, 988–991.
- Gayet, C.; Bailhache, E.; Dumon, H.; Martin, L.; Siliart, B.; Nguyen, P. Insulin resistance and changes in plasma concentration of TNFalpha, IGF1, and NEFA in dogs during weight gain and obesity. J. Anim. Physiol. Anim. Nutr. 2004, 88, 157–165.
- Veiga, A.P.M.; Price, C.A.; de Oliveira, S.T.; dos Santos, A.P.; Campos, R.; Barbosa, P.R.; Gonzalez, F.H.D. Association of canine obesity with reduced serum levels of C-reactive protein. J. Vet. Diagn. Investig. 2008, 20, 224–228.
- Van de Velde, H.; Janssens, G.P.; Stuyven, E.; Cox, E.; Buyse, J.; Hesta, M. Short-term increase of body weight triggers immunological variables in dogs. Vet. Immunol. Immunopathol. 2012, 145, 431–437.
- Van de Velde, H.; Janssens, G.P.; Rochus, K.; Duchateau, L.; Scharek-Tedin, L.; Zentek, J.; Nguyen, P.; Cox, E.; Buyse, J.; Biourge, V.; et al. Proliferation capacity of T-lymphocytes is affected transiently after a long-term weight gain in Beagle dogs. Vet. Immunol. Immunopathol. 2013, 152, 237–244.
- Frank, L.; Mann, S.; Levine, C.B.; Cummings, B.P.; Wakshlag, J.J. Increasing body condition score is positively associated interleukin-6 and monocyte chemoattractant protein-1 in Labrador retrievers. Vet. Immunol. Immunopathol. 2015, 167, 104–109.
- Piantedosi, D.; Di Loria, A.; Guccione, J.; De Rosa, A.; Fabbri, S.; Cortese, L.; Carta, S.; Ciaramella, P. Serum biochemistry profile, inflammatory cytokines, adipokines and cardiovascular findings in obese dogs. Vet. J. 2016, 216, 72–78.
- Ehrlich, A.; Moreno Castilho, T.; Goldsmith-Pestana, K.; Chae, W.J.; Bothwell, A.L.; Sparwasser, T.; McMahon-Pratt, D. The immunotherapeutic role of regulatory T cells in Leishmania (Viannia) panamensis infection. J. Immunol. 2014, 193, 2961–2970.
- Cortese, L.; Annunziatella, M.; Palatucci, A.T.; Rubino, V.; Piantedosi, D.; Di Loria, A.; Ruggiero, G.; Ciaramella, P.; Terrazzano, G. Regulatory T cells, Cytotoxic T lymphocytes and a T(H)1 cytokine profile in dogs naturally infected by Leishmania infantum. Res. Vet. Sci. 2013, 95, 942–949.
- Adalid-Peralta, L.; Fragoso, G.; Fleury, A.; Sciutto, E. Mechanisms underlying the induction of regulatory T cells and its relevance in the adaptive immune response in parasitic infections. Int. J. Biol. Sci. 2011, 7, 1412–1426.
- Di Loria, A.; Squillacioti, C.; De Luca, A.; Veneziano, V.; Mirabella, N.; Guccione, J.; Santoro, D. Increased leptin mRNA expression in the blood of dogs naturally infected by Leishmania infantum. Vet. J. 2014, 202, 634–636.
This entry is offline, you can click here to edit this entry!
