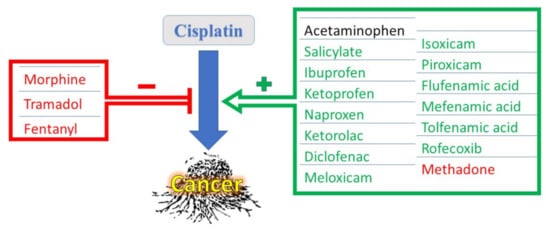
Cisplatin (CDDP), one of the most eminent cancer chemotherapeutic agents, has been successfully used to treat more than half of all known cancers worldwide. Despite its effectiveness, CDDP might cause severe toxic adverse effects on multiple body organs during cancer chemotherapy, including the kidneys, heart, liver, gastrointestinal tract, and auditory system, as well as peripheral nerves causing severely painful neuropathy. The latter, among other pains patients feel during chemotherapy, is an indication for the use of analgesics during treatment with CDDP. Different types of analgesics, such as acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDS), and narcotic analgesics, could be used according to the severity of pain. Administered analgesics might modulate CDDP’s efficacy as an anticancer drug. NSAIDS, on one hand, might have cytotoxic effects on their own and few of them can potentiate CDDP’s anticancer effects via inhibiting the CDDP-induced cyclooxygenase (COX) enzyme, or through COX-independent mechanisms. On the other hand, some narcotic analgesics might ameliorate CDDP’s anti-neoplastic effects, causing chemotherapy to fail. Concerning safety, some analgesics share the same adverse effects on normal tissues as CDDP, augmenting its potentially hazardous effects on organ impairment.
- cisplatin
- analgesics
- acetaminophen
- non-steroidal anti-inflammatory drugs
- morphine
- cytotoxicity
1. Interactions of NSAIDs with CDDP
1.1. Interactions of Salicylates with CDDP
1.2. Interaction of Propionic Acid-Derived NSAIDs (Profens) with CDDP
1.3. Interaction of Acetic Acid-Derived NSAIDS with CDDP
1.4. Interaction of Enolic Acid Derivatives of NSAIDs (Oxicams) with CDDP
1.5. Interaction of Anthranilic Acid and Naphthylalanine Derivatives of NSAIDs (Fenamates) with CDDP
1.6. Interaction of COX-II Selective NSAIDS (Coxibs) with CDDP
This entry is adapted from the peer-reviewed paper 10.3390/medicina58010046
