The tumor microenvironment (TME) exhibits unique characteristics that differ among various tumor types. It is composed of cancerous, non-cancerous, stromal, and immune cells that are surrounded and supported by components of the extracellular matrix (ECM). Therefore, the interactions among cancer cells, stromal cells, and components of the ECM determine cancer progression and response to therapy. Proteoglycans (PGs), hybrid molecules consisting of a protein core to which sulfated glycosaminoglycan chains are bound, are significant components of the ECM that are implicated in all phases of tumorigenesis. These molecules, secreted by both the stroma and cancer cells, are crucial signaling mediators that modulate the vital cellular pathways implicated in gene expression, phenotypic versatility, and response to therapy in specific tumor types. Specific inputs from the endocrine and immune systems are some of the characteristics of hormone-dependent cancer pathogenesis. Notably, the mechanisms involved in various aspects of cancer progression are executed in the ECM niche of the TME, and its' PG components crucially mediate these processes including cancer metastasis, angiogenesis, immunobiology, autophagy, and response to therapy.
- proteoglycans
- hormone-dependent cancers
- cancer metastasis
- tumor microenvironment
- chemoresistance
- biomarkers
- autophagy
- cancer imminobiology
Hormone-dependent cancers exhibit high morbidity and mortality. In spite of advances in therapy, the treatment of hormone-dependent cancers remains an unmet health need. The tumor microenvironment (TME) exhibits unique characteristics that differ among various tumor types. It is composed of cancerous, non-cancerous, stromal, and immune cells that are surrounded and supported by components of the extracellular matrix (ECM). Therefore, the interactions among cancer cells, stromal cells, and components of the ECM determine cancer progression and response to therapy. Proteoglycans (PGs), hybrid molecules consisting of a protein core to which sulfated glycosaminoglycan chains are bound, are significant components of the ECM that are implicated in all phases of tumorigenesis. These molecules, secreted by both the stroma and cancer cells, are crucial signaling mediators that modulate the vital cellular pathways implicated in gene expression, phenotypic versatility, and response to therapy in specific tumor types. A plethora of deregulated signaling pathways contributes to the growth, dissemination, and angiogenesis of hormone-dependent cancers. Specific inputs from the endocrine and immune systems are some of the characteristics of hormone-dependent cancer pathogenesis. Importantly, the mechanisms involved in various aspects of cancer progression are executed in the ECM niche of the TME, and the PG components crucially mediate these processes.
1. Introduction
All tumor types develop a unique tumor microenvironment (TME) that boasts different compositions of cancerous, non-cancerous, stromal, and immune cells in each phase of cancer progression. The different cell subtypes of TME interact with each other but also with components of the extracellular matrix (ECM) surrounding the cells [1] The ECM is a crucial regulator of all cellular functions and a significant component of the TME. Importantly, ECM cues coordinate the different effectors of the TME and modulate the plethora of signaling pathways involved in the pathogenesis of cancer [2][3]. Even early reports showed that desmoplasia, or an accumulation of the ECM, is a characteristic property of tumors, and increased ECM contents are frequently associated with dismal prognosis in various tumor types [4]. Proteoglycans (PGs) are significant components of the ECM implicated in all phases of tumorigenesis. Their “hybrid” composition, consisting of a protein core and glycosaminoglycan (GAG) chains, bestows these molecules with high versatility and ability to interact with many cellular effectors [5]. Modifications in PG content and structure are correlated with disease progression in various cancer types. Importantly, PGs, like other components of the ECM, are secreted by both stroma (e.g., cancer-associated fibroblasts) and cancer cells [6]. Of note, PGs are crucial regulators of the bioavailability of growth factors, hormones, and cytokines as well as the resulting activation of their respective receptors that modify gene expression, phenotypic versatility, and response to therapy in specific tumor types [7]. Recent advances in omics technologies have shown that PGs are among the molecules whose gene signature is predictive of cancer development and prognosis [8].
Hormone-dependent cancers exhibit high morbidity and mortality. In spite of advances in therapy, the treatment of hormone-dependent cancers remains an unmet health need. Hormones are vital signaling molecules that are produced by glands and play a crucial role in regulating body physiology and pathophysiology [9]. These active mediators, such as androgens and estrogens, can control cell behavior by binding to specific receptor proteins in the target cell [10]. Their critical role in cell signaling gives hormones the ability to deregulate the functions of target cells under certain conditions and, thus, to promote a cancerous phenotype.
Two of the most common solid malignancies that are sex- and hormone-dependent are breast cancer (BC) and prostate cancer (PC). A plethora of deregulated signaling pathways contributes to the growth, dissemination, and angiogenesis of these tumors [11][12].
Various mechanisms have been found to be correlated to resistance in hormone-dependent cancers, with specific differences exhibited between BC and PC. There is evidence that immunological responses to foreign and self-antigens are sex-dependent, and there are differences in innate and adaptive immune responses. These sex hormone-related changes to immunity may be associated with different immunoediting in hormone-dependent cancer and explain the differential susceptibility of males and females to malignancies [13][14]. Autophagy and apoptosis have been correlated to chemoresistance and cancer stem cell (CSC) properties [15][16]. Importantly, the mechanisms involved in various aspects of cancer progression are executed in the ECM niche of the TME, and the ECM components crucially mediate these processes. Here, we comprehensively discuss the mechanisms through which PGs affect the multifaceted aspects of hormone-dependent cancer development and progression. Determining the individualized role of PGs in cancer patients can lead to new therapeutical strategies in the form of adjuvants or treatments to replace the standard therapy protocols.
2. Proteoglycans
PGs are composed of a protein core into which one or more GAG chains are covalently bound [17]. The GAG chains consist of repeated disaccharide units and are negatively charged [18]. The disaccharide building blocks consist of an amino sugar (glucosamine that is N-acetylated or N-sulfated or N-acetylgalactosamine) and uronic acid (glucuronic acid or iduronic acid) or galactose . There are four different types of GAG chains, usually glycosylate PGs, e.g., heparan sulfate (HS), chondroitin sulfate/dermatan sulfate (CS/DS), or keratan sulfate (KS) . Serglycin is the only known PG which can be decorated with the GAG heparin [19]. CS/DS, HS, and heparin bind to the protein core through a specific trisaccharide linker composed of two galactose (Gal) and one xylose (Xyl) residue. The linker explicitly binds to a serine residue of the protein core via an O-glycosidic bond, creating the final structure GAG-GalGalXyl-O-CH2-protein [20].KS is a sulfated poly-N-acetylgalactosamine chain substituted on a limited number of PGs as an N-linked or O-linked chain [5].The type of GAG substitution classifies the 45 PG members as chondroitin sulfate PGs (CSPGs), heparan sulfate PGs (HSPGs), or keratan sulfate PGs (KSPGs). The properties of PGs are dependent on both the protein core and the GAG substitution [21].
Moreover, PGs can be glycosylated by more than one kind of GAG [22][23]. GAG chains can interact with a wide variety of signaling macromolecules to regulate fundamental biological processes and, thus, bestow PGs with a plethora of structural and functional variations . The glycosylation of PGs is initiated in the endoplasmic reticulum, further orchestrated in the Golgi apparatus, and is mostly mediated by glycosyltransferases that reside in this complex organelle [24].
The PGs can be classified based on their location as intracellular (e.g., serglycin), at the cell membrane (e.g., betaglycan), pericellular (e.g., perlecan), and away from the cell or “extracellular” (e.g., biglycan). The small leucine-rich proteoglycans (SLRPs) are a prominent component of extracellular PGs, comprising the largest class of PGs [22]. Serglycin is the only intracellular PG, initially identified in granules of mast cells, where it stabilizes proteases that are released upon inflammation [25]. However, in continuation, it was shown that the majority of immune cells express serglycin and store it within intracytoplasmic granules to control the bioactivity of various inflammatory mediators like chemokines or cytokines [26].
Hyalectans are another family of extracellular PGs. This family compromises four PGs, namely aggrecan, versican, neurocan, and brevican, which are endowed with distinct similarities at both the genomic and protein levels [27].All four hyalectan members share a tri-domain structure consisting of an N-terminal domain with affinity towards hyaluronan, a central domain decorated with CS chains, and a C-terminal region with an ability to bind lectins [27]. Due to alternative splicing and variability in the level of glycosylation, these PGs exert different operating properties with the ability to function as molecular bridges between cell surfaces and extracellular molecular networks . Aggrecan, in common with other hyalectan members, has the ability to aggregate into large supramolecular complexes and is the principal load-bearing PG component of cartilage [28]. Versican is the largest hyalectan member when expressed as the full-length isoform V0 . Versican isoforms, the result of discrete splicing, exhibit high variability in tissue expression correlated to complex roles in vital cellular functions, including adhesion, migration, and inflammation [29]. Neurocan and brevican are important hyalectans of the brain involved in the regulation of neural axon outgrowth and the biology of neural stem cells, among other processes [30][31][32][33].
As regarding cell surface PGs, thirteen genes encode these proteins. Of these, seven are transmembrane PGs, e.g., syndecans, while the remaining six are glypicans, glycosyl phosphoinositol (GPI)-anchored PGs [17]. These PGs mainly contribute to cell migration and adhesion processes as their intracellular domain connects the cytoskeleton and kinases with the extracellular environment [34]. Moreover, transmembrane PGs interact with or serve as coreceptors for many signaling molecules, such as growth factors or Wnt proteins. In this manner, they have the ability to enhance or inhibit signaling pathways, affecting their respective cell functions [35][36]. Pericellular PGs are also found anchored on the cell surface of different cell types via integrins or cell surface receptors. These PGs are mostly HSPGs, and their HS chains can be cleaved by various enzymes and act as pro-angiogenic factors [37]. The largest PG category is the extracellular PGs, which are structural components of the ECM that have a supportive role for the cells in tissues, but also act as signaling mediators.
SLRPs are defined as PGs with a relatively small protein core (36–42 kDa) harboring multiple leucine-rich repeats (LRRs) and substituted with GAG chains [38]. Initially, the SLRPs were classified into three distinct classes based on the conservation of the amino acid residues of their protein cores, the organization of disulfide bonds at the N- and C-terminal regions of the molecule, and their genomic organization/gene homology [39][40][41]. In continuation, the SLRPs gene family has been expanded to include 18 genes classified into five separate subfamilies [42]. Many studies have shown that these PGs bind with biologic mediators, including growth factors, to participate in cells’ interactions with their microenvironment [43][44]. Indeed, the SLRPs act as signaling molecules that participate in the regulation of basal cellular functions, like proliferation, migration, and differentiation. The SLRPs can interact with tyrosine kinase receptors, and this is one of their primary mechanisms of action through which they regulate downstream intracellular signaling and, ultimately, cellular behavior [45][46][47][48].
3. Proteoglycan Expression in Hormone-Dependent Cancer
It is well established that PGs take part in signaling pathways that control cellular functions. PGs’ expression and glycosylation profiles differ between normal and malignant tissues, a fact that affects cell behavior and differentiation status.
The majority of BC cells express estrogen receptor-α (ER-α), a diagnostic biomarker for this malignancy, while the role of the second ER isoform, ERβ, expressed by the mammary gland, is yet to be defined in BC. Estrogens regulate the functions of the female reproductive system by binding to these specific receptors [49]. ER-α is also expressed by cancerous prostate cells, while, respectively to estrogens, androgens are the male sex hormones that bind to androgen receptors (AR) and are expressed during all stages of prostate carcinogenesis and related to hereditary PC [50][51].
PGs show discrete expression patterns between normal and tumor tissues. Thus, Suhovskih et al. showed that the expression profiles of PGs glypican-1, perlecan, syndecan-1, aggrecan, versican, NG2, brevican, decorin, and lumican differ between healthy prostate tissue and prostate tumors [52]. Indeed, it was demonstrated that versican, decorin, and biglycan were prevalent PGs in normal prostate tissue stroma, whereas syndecan-1 and glypican-1 were localized to the epithelial component. In prostate tumor tissues, a marked attenuation of total decorin and lumican expression was evident, whereas syndecan-1 and glypican-1 expression was increased in tumor stroma and attenuated in tumor epithelial cells [52]. Indeed, the downregulation of syndecan-1 expression in PC was associated with the aggressive behavior of these cells [53] and clinical tumor progression [54]. Syndecan-2 was found to be expressed by PC cells and not expressed by normal prostate epithelial cells [55]. Syndecan-1 and -2 expression by PC cells was proposed as a useful prognostic marker for patients who presented with clinically localized cancer. In combination with prostate-specific antigen, evaluation of the expression of these PGs resulted in improved assessment of the risk of recurrence [56].
On the other hand, the expression of lumican in the stroma circumjacent to primary prostate tumors was found to attenuate the progression of this malignancy [57]. Recently, it was shown that the SLRP fibromodulin has discrete expression among PC and benign tissues [58]. PC tissues express this PG, as do PC cell lines with, interestingly, the highest expression determined in the androgen-sensitive human metastatic prostate adenocarcinoma LNCaP clone [59]. Moreover, the glypican-1 expression in urine cell sediments was proposed as a possible positive marker for PC [60]. The low expression of another glypican member, glypican-5, has, on the other hand, been correlated to PC progression [61].
It is worth noting that early studies have shown that androgens affect the expression of PGs in the prostate gland [62], and that the effects differed between wild type and castrated rats [63]. These authors suggested that the effects of androgens on prostatic growth are partly dependent on the interactions between the parenchyma and stroma facilitated by PGs [63]. Moreover, the GAG component differs among benign and malignant prostate tissues, and GAGs, along with other glycan types, have been suggested as biomarkers for PC [64]. Edwards, in 2012, discussed that in PC, decorin, which also interacts with TGF-b [65][66], and betaglycan inhibit tumor growth and metastasis, while versican and perlecan respectively promote cell motility and invasion, and tumor cell growth and angiogenesis.
Indeed, it is suggested that PGs are a significant venue of communication between normal prostate epithelial cells and fibroblasts, and changes in PG expression by cancer cells contribute to the deregulation of cell–cell contact growth inhibition [67].
PGs have likewise been indicated as significant mediators of BC tumorigenesis [68]. Mammographic density mirrors alterations in the composition of breast tissue as distinguished on a mammogram and is immediately correlated to the dominance of epithelial and stromal components [69]. Histologically, the stroma is the region exhibiting the highest mammographic density [70]. Importantly, mammographic density is immediately associated with the protein, including PG content, of the breast tissue [71]. PGs are discretely expressed in normal breast tissue depending on hormone status, whereas there are significant alterations of PGs in BC tissues. One example is the pericellular PG perlecan, whose expression is significantly attenuated in BC compared with in healthy tissue [72]. These changes in mammographic density, dependent on PGs, are attributed to their ability to retain water [73].
HSPGs participate in the development of the mammary gland, during which substantial changes in mammographic density are noted [74]. Indeed, mammographic density is an essential independent BC risk factor and is correlated to other various BC risk factors. Therefore, Hanna and Diorio suggest that it is significant to examine the correlation of the expression of individual proteins with changes in mammographic density and evaluate their correlation with BC risk . The expression pattern of syndecan-1 is altered during BC tumorigenesis [75]. It is expressed in the stroma of the BC and by the epithelium of normal breast tissue[76]. Indeed, it is suggested that the redistribution of syndecan-1 leads to significantly increased mammographic density, and its total expression is higher in the dense as compared to non-dense breast tissue of postmenopausal women [77]. Moreover, the expression of syndecan-1 was found to be lower in normal breast tissue during luteal as compared to the follicular phase of the menstrual cycle [78]. These data demonstrate that the expression of syndecan-1 is directly dependent on estrogen levels, which increase during the follicular phase of the menstrual cycle and are correlated to the transient changes in the density of breast tissue stroma [78].
Syndecan-2 was found to be expressed by BC cell lines [79] and to facilitate the ability of BC cells to invade [80]. The expression of syndecan-4 seems to attenuate the aggressive behavior of BC cells as it neutralizes the pro-invasive properties of syndecan-2 [81].
The expression of glypicans is also modified in BC tissues. Indeed, the expression of glypican-1 is found to be significantly upregulated in BC and was determined to affect the action of heparin-binding growth factors [82]. By contrast, glypican-3 is downregulated [83] and attenuates the metastasis of BC cells in a syngeneic BC model [84].
The expression of the class I SLRPs biglycan and decorin as well as of enzymes implicated in PGs synthesis was found to be modified in BC. Indeed, the unique identified pattern of their expression was determined to have clinical relevance [85]. An early report, however, suggested that the expression of biglycan is low in both normal and BC tissues [86]. Interestingly, the expression of biglycan was shown to be enhanced in precancerous lesions [87].
Lumican, an SLRP also implicated in tumorigenesis , is the main SLRP expressed by normal and cancerous breast tissue [88]. The effects of lumican on BC pathogenesis are controversial. Early reports indicate that lumican is mostly expressed by BC stroma cells and was positively correlated with higher tumor grade, lower expression of ERs, as well as to the younger age of patients [89]. Furthermore, an increased expression of lumican and decorin correlated to enhanced mammographic density was shown in benign and precancerous breast lesions [90]. Specific lumican polymorphisms were associated with BC risk. Thus, LUM rs2268578 was correlated with ER-positive BC in the Mayo Clinic study, whereas a modest association was detected in the Studies of Epidemiology and Risk Factors in Cancer Heredity SEARCH sample [91].
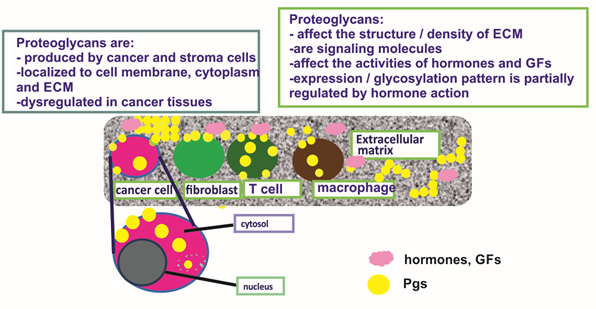
BC tissues express the large CS-containing PGs, hyalectans such as versican [92]. Indeed, all known isoforms of versican are overexpressed in the malignant lesions, both at the protein and mRNA levels [93]. These authors had also detected a novel alternatively spliced versican isoform (V4) whose expression was enhanced in human BC [93]. A separate study showed high versican expression in the perilesional stroma of a specific subclass of ductal in situ carcinomas, and that this expression pattern was correlated to the high grade (G3) category of the tumor [94]. Canavese et al., however, did not detect changes in the expression of versican in lobular in situ carcinoma as compared to normal breast tissue [94]. Furthermore, it was shown that versican levels were increased in cancer tissues exhibiting malignant-appearing microcalcifications as compared to normal breast tissues. On the other hand, the hyalectan, aggrecan transcription levels were not altered in BC tissues [95]. The expression and critical roles of PGs in hormone-dependent cancer are concisely depicted in Figure 1.
Figure 1. The expression and critical roles of proteoglycans (PGs) in hormone-dependent cancer. PGs are expressed at the cell membrane and cytosol of cancer and stroma cells and secreted to the extracellular matrix (ECM). They contribute to the structure of cancer tissues, e.g., the regulation of tissue density and affect the activities of growth factors and hormones.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12092401
References
- Yu, Y.-R.; Ho, P.-C. Sculpting tumor microenvironment with immune system: From immunometabolism to immunoediting. Clin. Exp. Immunol. 2019, 197, 153–160, doi:10.1111/cei.13293.
- Tzanakakis, G.; Neagu, M.; Tsatsakis, A.; Nikitovic, D. Proteoglycans and Immunobiology of Cancer—Therapeutic Implications. Front. Immunol. 2019, 10, 875, doi:10.3389/fimmu.2019.00875.
- Nikitovic, D.; Tzardi, M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N. Cancer Microenvironment and Inflammation: Role of Hyaluronan. Front. Immunol. 2015, 6, 169, doi:10.3389/fimmu.2015.00169.
- Anastassiades, O.T.; Pryce, D.M. Fibrosis as an Indication of Time in Infiltrating Breast Cancer and its Importance in Prognosis. Br. J. Cancer 1974, 29, 232–239, doi:10.1038/bjc.1974.62.
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Essentials of Glycobiology. In Proteoglycans and Sulfated Glycosaminoglycans, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015–2017.
- Naba, A.; Clauser, K.R.; Lamar, J.M.; A Carr, S.; O Hynes, R. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife 2014, 3, e01308, doi:10.7554/elife.01308.
- Nikitovic, D.; Papoutsidakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Lumican affects tumor cell functions, tumor–ECM interactions, angiogenesis and inflammatory response. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 35, 206–214, doi:10.1016/j.matbio.2013.09.003.
- Yuzhalin, A.E.; Urbonas, T.; A Silva, M.; Muschel, R.J.; Gordon-Weeks, A.N. A core matrisome gene signature predicts cancer outcome. Br. J. Cancer 2018, 118, 435–440, doi:10.1038/bjc.2017.458.
- Heyland, A.; Hodin, J.; Reitzel, A.M. Hormone signaling in evolution and development: A non-model system approachs. BioEssays News Rev. Mol. Cell. Dev. Biol. 2005, 27, 64–75, doi:10.1002/bies.20136.
- Ruhs, S.; Nolze, A.; Hübschmann, R.; Grossmann, C. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Nongenomic effects via the mineralocorticoid receptor. J. Endocrinol. 2017, 234, T107–T124, doi:10.1530/joe-16-0659.
- Cheng, M.; Michalski, S.; Kommagani, R. Role for Growth Regulation by Estrogen in Breast Cancer 1 (GREB1) in Hormone-Dependent Cancers. Int. J. Mol. Sci. 2018, 19, 2543, doi:10.3390/ijms19092543.
- Subramani, R.; Nandy, S.B.; Pedroza, D.A.; Lakshmanaswamy, R. Role of Growth Hormone in Breast Cancer. Endocrinology 2017, 158, 1543–1555, doi:10.1210/en.2016-1928.
- Bupp, M.R.G.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, doi:10.3389/fimmu.2018.01269.
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638, doi:10.1038/nri.2016.90.
- Zheng, H.-C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964, doi:10.18632/oncotarget.19048.
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75, doi:10.1016/bs.acr.2017.11.001.
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 42, 11–55, doi:10.1016/j.matbio.2015.02.003.
- Raman, R.; Sasisekharan, V.; Sasisekharan, R. Structural Insights into Biological Roles of Protein-Glycosaminoglycan Interactions. Chem. Biol. 2005, 12, 267–277, doi:10.1016/j.chembiol.2004.11.020.
- Kolset, S.O., Tveit, H. Serglycin--structure and biology. Cell Mol. Life Sci.2008, 65, 1073–1085.
- Gandhi, N.S.; Mancera, R.L. The Structure of Glycosaminoglycans and their Interactions with Proteins. Chem. Biol. Drug Des.2008, 72, 455–482, doi:10.1111/j.1747-0285.2008.00741.x
- Nikitovic, D.; Mytilinaiou, M.; Berdiaki, A.; Karamanos, N.K.; Tzanakakis, G.N. Heparan sulfate proteoglycans and heparin regulate melanoma cell functions. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 2471–2481, doi:10.1016/j.bbagen.2014.01.031.
- Listik, E.; Gaschler, J.A.M.; Matias, M.; Feres, M.F.N.; Toma, L.; Nahás-Scocate, A.C.R.; Gascheler, J.A.M. Proteoglycans and dental biology: The first review. Carbohydr. Polym. 2019, 225, 115199, doi:10.1016/j.carbpol.2019.115199.
- Nikitovic, D.; Assouti, M.; Sifaki, M.; Katonis, P.; Krasagakis, K.; Karamanos, N.K.; Tzanakakis, G.N. Chondroitin sulfate and heparan sulfate-containing proteoglycans are both partners and targets of basic fibroblast growth factor-mediated proliferation in human metastatic melanoma cell lines. Int. J. Biochem. Cell Biol. 2008, 40, 72–83, doi:10.1016/j.biocel.2007.06.019.
- Akintayo, A.; Stanley, P. Roles for Golgi Glycans in Oogenesis and Spermatogenesis. Front. Cell Dev. Biol. 2019, 7, 98, doi:10.3389/fcell.2019.00098.
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv. Immunol. 2014, 122, 211–252, doi:10.1016/B978-0-12-800267-4.00006-7.
- Korpetinou, A.; Skandalis, S.S.; Labropoulou, V.T.; Smirlaki, G.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Serglycin: At the Crossroad of Inflammation and Malignancy. Front. Oncol. 2014, 3, 327, doi:10.3389/fonc.2013.00327.
- Iozzo, R.V. MATRIX PROTEOGLYCANS: From Molecular Design to Cellular Function. Annu. Rev. Biochem. 1998, 67, 609–652, doi:10.1146/annurev.biochem.67.1.609.
- Wight, T.N.; Toole, B.P.; Hascall, V.C. Hyaluronan and the Aggregating Proteoglycans. In The Extracellular Matrix: An Overview; Mecham, R.P., Ed.; Springer: Berlin, Germany, 2011; pp. 147–195.
- Wight, T.N.; Kinsella, M.G.; Evanko, S.P.; Potter-Perigo, S.; Merrilees, M.J. Versican and the regulation of cell phenotype in disease. Biochim. Biophys. Acta BBA Bioenerg. 2014, 1840, 2441–2451, doi:10.1016/j.bbagen.2013.12.028.
- Yamaguchi, Y. Brevican: A major proteoglycan in adult brain. Perspect. Dev. Neurobiol. 1996, 3, 307–317.
- Liu, B.P.; Cafferty, W.B.; O Budel, S.; Strittmatter, S.M. Extracellular regulators of axonal growth in the adult central nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1593–1610, doi:10.1098/rstb.2006.1891.
- Theocharidis, U.; Long, K.; Ffrench-Constant, C.; Faissner, A. Regulation of the neural stem cell compartment by extracellular matrix constituents. Prog. Brain Res. 2014, 214, 3–28, doi:10.1016/b978-0-444-63486-3.00001-3.
- Henrich-Noack, P.; Nikitovic, D.; Neagu, M.; Docea, A.O.; Engin, A.B.; Gelperina, S.; Shtilman, M.; Mitsias, P.; Tzanakakis, G.; Gozes, I.; et al. The blood–brain barrier and beyond: Nano-based neuropharmacology and the role of extracellular matrix. Nanomedicine 2019, 17, 359–379, doi:10.1016/j.nano.2019.01.016.
- Couchman, J.R. Transmembrane Signaling Proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114, doi:10.1146/annurev-cellbio-100109-104126.
- Mytilinaiou, M.; Nikitovic, D.; Berdiaki, A.; Kostouras, A.; Papoutsidakis, A.; Tsatsakis, A.; Tzanakakis, G.N. Emerging roles of syndecan 2 in epithelial and mesenchymal cancer progression. IUBMB Life 2017, 69, 824–833, doi:10.1002/iub.1678.
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480.
- Reiland, J.; Sanderson, R.D.; Waguespack, M.; Barker, S.A.; Long, R.; Carson, D.D.; Marchetti, D. Heparanase Degrades Syndecan-1 and Perlecan Heparan Sulfate: Functional implications for tumor cell invasion. J. Biol. Chem. 2003, 279, 8047–8055, doi:10.1074/jbc.m304872200.
- Iozzo, R.V.; Murdoch, A.D. Proteoglycans of the extracellular environment: Clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1996, 10, 598–614, doi:10.1096/fasebj.10.5.8621059.
- Iozzo, R.V. The Family of the Small Leucine-Rich Proteoglycans: Key Regulators of Matrix Assembly and Cellular Growth. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 141–174, doi:10.3109/10409239709108551.
- Danielson, K.G.; Fazzio, A.; Cohen, I.; Cannizzaro, L.A.; Eichstetter, I.; Iozzo, R.V. The human decorin gene: Intron-exon organization, discovery of two alternatively spliced exons in the 5′ untranslated region, and mapping of the gene to chromosome 12q23. Genomics 1993, 15, 146–160.
- Nikitovic, D.; Berdiaki, K.; Chalkiadaki, G.; Karamanos, N.K.; Tzanakakis, G. The Role of SLRP-Proteoglycans in Osteosarcoma Pathogenesis. Connect. Tissue Res. 2008, 49, 235–238, doi:10.1080/03008200802147589.
- Schaefer, L.; Iozzo, R.V. Biological Functions of the Small Leucine-rich Proteoglycans: From Genetics to Signal Transduction. J. Biol. Chem. 2008, 283, 21305–21309, doi:10.1074/jbc.r800020200.
- Nikitovic, D.; Aggelidakis, J.; Young, M.F.; Iozzo, R.V.; Karamanos, N.K.; Tzanakakis, G.N. The Biology of Small Leucine-rich Proteoglycans in Bone Pathophysiology. J. Biol. Chem. 2012, 287, 33926–33933, doi:10.1074/jbc.r112.379602.
- Schaefer, L.; Tredup, C.; Gubbiotti, M.A.; Iozzo, R.V. Proteoglycan neofunctions: Regulation of inflammation and autophagy in cancer biology. FEBS J. 2016, 284, 10–26, doi:10.1111/febs.13963.
- Zafiropoulos, A.; Nikitovic, D.; Katonis, P.; Tsatsakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Decorin-Induced Growth Inhibition Is Overcome through Protracted Expression and Activation of Epidermal Growth Factor Receptors in Osteosarcoma Cells. Mol. Cancer Res. 2008, 6, 785–794, doi:10.1158/1541-7786.mcr-07-0165.
- Voudouri, K.; Nikitovic, D.; Berdiaki, A.; Kletsas, D.; Karamanos, N.K.; Tzanakakis, G.N. IGF-I/EGF and E2 signaling crosstalk through IGF-IR conduit point affects breast cancer cell adhesion. Matrix Biol. J. Int. Soc. Matrix Biol. 2016, 56, 95–113, doi:10.1016/j.matbio.2016.06.005.
- Aggelidakis, J.; Berdiaki, A.; Nikitovic, D.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.M.; Tzanakakis, G.N. Biglycan Regulates MG63 Osteosarcoma Cell Growth Through a LPR6/beta-Catenin/IGFR-IR Signaling Axis. Front. Oncol. 2018, 8, 470.
- Papoutsidakis, A.; Giatagana, E.M.; Berdiaki, A.; Spyridaki, I.; Spandidos, D.A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican mediates HTB94 chondrosarcoma cell growth via an IGF-IR/Erk1/2 axis. Int. J. Oncol. 2020, in press.
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170, doi:10.1016/bs.apcsb.2019.01.001.
- Grozescu, T.; Popa, F. Prostate cancer between prognosis and adequate/proper therapy. J. Med. Life 2017, 10, 5–12.
- Jozwik, K.M.; Carroll, J.S. Pioneer factors in hormone-dependent cancers. Nat. Rev. Cancer 2012, 12, 381–385, doi:10.1038/nrc3263.
- Suhovskih, A.V.; Mostovich, L.A.; Kunin, I.S.; Boboev, M.M.; Nepomnyashchikh, G.I.; Aidagulova, S.V.; Grigorieva, E.V. Proteoglycan Expression in Normal Human Prostate Tissue and Prostate Cancer. ISRN Oncol. 2013, 2013, 1–9, doi:10.1155/2013/680136.
- Farfán, N.; Ocarez, N.; Castellón, E.A.; Mejía, N.; De Herreros, A.G.; Contreras, H.R. The transcriptional factor ZEB1 represses Syndecan 1 expression in prostate cancer. Sci. Rep. 2018, 8, 11467, doi:10.1038/s41598-018-29829-1.
- Kiviniemi, J.; Kallajoki, M.; Kujala, I.; Matikainen, M.-T.; Alanen, K.; Jalkanen, M.; Salmivirta, M. Altered expression of syndecan-1 in prostate cancer. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2004, 112, 89–97, doi:10.1111/j.1600-0463.2004.apm1120202.x.
- Popovic, A.; Demirović, A.; Spajić, B.; Štimac, G.; Krušlin, B.; Tomas, D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis. 2009, 13, 78–82, doi:10.1038/pcan.2009.43.
- Ledezma, R.; Cifuentes, F.; Gallegos, I.; Fullá, J.; Ossandón, E.; A Castellón, E.; Contreras, H.R. Altered expression patterns of syndecan-1 and -2 predict biochemical recurrence in prostate cancer. Asian J. Androl. 2011, 13, 476–480, doi:10.1038/aja.2010.143.
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; De Paula, C.A.A.; Carneiro, C.R.; Ortiz, V.; Toma, L.; Kao, W.W.-Y.; Nader, H.B. Lumican expression, localization and antitumor activity in prostate cancer. Exp. Cell Res. 2013, 319, 967–981, doi:10.1016/j.yexcr.2013.01.023.
- Bettin, A.; Reyes, I.; Reyes, N. Gene Expression Profiling of Prostate Cancer–Associated Genes Identifies Fibromodulin as Potential Novel Biomarker for Prostate Cancer. Int. J. Biol. Markers 2016, 31, 153–162, doi:10.5301/jbm.5000184.
- Reyes, N.; Benedetti, I.; Bettin, A.; Rebollo, J.; Geliebter, J. The small leucine rich proteoglycan fibromodulin is overexpressed in human prostate epithelial cancer cell lines in culture and human prostate cancer tissue. Cancer Biomark. Sect. A Dis. Markers 2016, 16, 191–202, doi:10.3233/cbm-150555.
- Campbell, D.H.; Lund, M.E.; Nocon, A.L.; Cozzi, P.J.; Frydenberg, M.; De Souza, P.; Schiller, B.; Beebe-Dimmer, J.L.; Ruterbusch, J.J.; Walsh, B. Detection of glypican-1 (GPC-1) expression in urine cell sediments in prostate cancer. PLoS ONE 2018, 13, e0196017, doi:10.1371/journal.pone.0196017.
- Zhang, C.; Liu, Z.; Wang, L.; Qiao, B.; Du, E.; Li, L.; Xu, Y.; Zhang, Z. Prognostic significance of GPC5 expression in patients with prostate cancer. Tumor Biol. 2015, 37, 6413–6418, doi:10.1007/s13277-015-4499-3.
- Terry, D.E.; Clark, A.F. Influence of testosterone on chondroitin sulphate proteoglycan in the rat prostate. Biochem. Cell Biol. 1996, 74, 645–651, doi:10.1139/o96-069.
- Kofoed, J.A.; Tumilasci, O.R.; Curbelo, H.M.; Lemos, S.M.F.; Arias, N.H.; Houssay, A.B. Effects of castration and androgens upon prostatic proteoglycans in rats. Prostate 1990, 16, 93–102, doi:10.1002/pros.2990160202.
- Scott, E.; Munkley, J. Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1389, doi:10.3390/ijms20061389.
- Edwards, I.J. Proteoglycans in prostate cancer. Nat. Rev. Urol. 2012, 9, 196–206, doi:10.1038/nrurol.2012.19.
- Coulson-Thomas, V.J.; Gesteira, T.F.; Coulson-Thomas, Y.M.; Vicente, C.M.; Tersariol, I.L.S.; Nader, H.B.; Toma, L. Fibroblast and prostate tumor cell cross-talk: Fibroblast differentiation, TGF-β, and extracellular matrix down-regulation. Exp. Cell Res. 2010, 316, 3207–3226, doi:10.1016/j.yexcr.2010.08.005.
- Suhovskih, A.V.; Kashuba, V.I.; Klein, G.; Grigorieva, E.V. Prostate cancer cells specifically reorganize epithelial cell-fibroblast communication through proteoglycan and junction pathways. Cell Adhes. Migr. 2016, 11, 39–53, doi:10.1080/19336918.2016.1182292.
- Theocharis, A.D.; Skandalis, S.S.; Neill, T.; Multhaupt, H.A.B.; Hubo, M.; Frey, H.; Gopal, S.; Gómes, A.; Afratis, N.; Lim, H.C.; et al. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochim. Biophys. Acta BBA Bioenerg. 2015, 1855, 276–300, doi:10.1016/j.bbcan.2015.03.006.
- Hanna, M.; Diorio, C. Does mammographic density reflect the expression of breast cancer markers? Climacteric J. Int. Menopause Soc. 2013, 16, 407–416.
- Britt, K.; Ingman, W.; Huo, C.; Chew, G.; Thompson, E. The pathobiology of mammographic density. J. Cancer Biol Res. 2014, 2, 1021–1031.
- Shawky, M.S.; Ricciardelli, C.; Lord, M.S.; Whitelock, J.; Ferro, V.; Britt, K.L.; Thompson, E.W. Proteoglycans: Potential Agents in Mammographic Density and the Associated Breast Cancer Risk. J. Mammary Gland. Biol. Neoplasia 2015, 20, 121–131, doi:10.1007/s10911-015-9346-z.
- Jansson, M.; Billing, O.; Herdenberg, C.; Lundin, C.; Tolockiene, E.; Nazemroaya, A.; Sund, M. Expression and Circulating Levels of Perlecan in Breast Cancer—Implications for Oestrogen Dependent Stromal Remodeling. J. Mammary Gland. Biol. Neoplasia 2020, 25, 69–77, doi:10.1007/s10911-020-09447-2.
- Stoeckelhuber, M.; Stumpf, P.; Hoefter, E.A.; Welsch, U. Proteoglycan–collagen associations in the non-lactating human breast connective tissue during the menstrual cycle. Histochem. Cell Biol. 2002, 118, 221–230, doi:10.1007/s00418-002-0438-7.
- Lteif, A.; Javed, A. Development of the Human Breast. Semin. Plast. Surg. 2013, 27, 005–012, doi:10.1055/s-0033-1343989.
- Löfgren, L.; Sahlin, L.; Jiang, S.; Von Schoultz, B.; Fernstad, R.; Skoog, L.; Von Schoultz, E. Expression of syndecan-1 in paired samples of normal and malignant breast tissue from postmenopausal women. Anticancer. Res. 2007, 27, 3045–3050.
- Maeda, T.; Alexander, C.M.; Friedl, A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 2004, 64, 612–621, doi:10.1158/0008-5472.can-03-2439.
- Lundström, E.; Sahlin, L.; Skoog, L.; Hagerstrom, T.; Svane, G.; Azavedo, E.; Sandelin, K.; Von Schoultz, B. Expression of syndecan-1 in histologically normal breast tissue from postmenopausal women with breast cancer according to mammographic density. Climacteric J. Int. Menopause Soc. 2006, 9, 277–282, doi:10.1080/13697130600865741.
- Hallberg, G.; Andersson, E.; Naessén, T.; Ekman-Ordeberg, G. The expression of syndecan-1, syndecan-4 and decorin in healthy human breast tissue during the menstrual cycle. Reprod. Biol. Endocrinol. 2010, 8, 35, doi:10.1186/1477-7827-8-35.
- Lim, H.C.; Multhaupt, H.A.B.; Couchman, J.R. Cell surface heparan sulfate proteoglycans control adhesion and invasion of breast carcinoma cells. Mol. Cancer 2015, 14, 15, doi:10.1186/s12943-014-0279-8.
- Lim, H.C.; Couchman, J.R. Syndecan-2 regulation of morphology in breast carcinoma cells is dependent on RhoGTPases. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 2482–2490, doi:10.1016/j.bbagen.2014.01.018.
- Kousidou, O.C.; Berdiaki, A.; Kletsas, D.; Zafiropoulos, A.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N. Estradiol–estrogen receptor: A key interplay of the expression of syndecan‐2 and metalloproteinase‐9 in breast cancer cells. Mol. Oncol. 2008, 2, 223–232, doi:10.1016/j.molonc.2008.06.002.
- Matsuda, K.; Maruyama, H.; Guo, F.; Kleeff, J.; Itakura, J.; Matsumoto, Y.; Lander, A.D.; Korc, M. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001, 61, 5562–5569.
- Xiang, Y.-Y.; Ladeda, V.; Filmus, J. Glypican-3 expression is silenced in human breast cancer. Oncogene 2001, 20, 7408–7412, doi:10.1038/sj.onc.1204925.
- Peters, M.G.; Farías, E.; Colombo, L.; Filmus, J.; Puricelli, L.; Joffé, E.B.D.K. Inhibition of Invasion and Metastasis by Glypican-3 in a Syngeneic Breast Cancer Model. Breast Cancer Res. Treat. 2003, 80, 221–232, doi:10.1023/a:1024549729256.
- Subbarayan, K.; Seliger, B. Tumor-dependent Effects of Proteoglycans and Various Glycosaminoglycan Synthesizing Enzymes and Sulfotransferases on Patients’ Outcome. Curr. Cancer Drug Targets 2019, 19, 210–221, doi:10.2174/1568009618666180706165845.
- Wadhwa, S.; Embree, M.C.; Bi, Y.; Young, M.F. Regulation, Regulatory Activities, and Function of Biglycan. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 16, doi:10.1615/critreveukargeneexpr.v14.i4.50.
- Van Bockstal, M.; Lambein, K.; Van Gele, M.; De Vlieghere, E.; Limame, R.; Braems, G.; Van den Broecke, R.; Cocquyt, V.; Denys, H.; Bracke, M.; et al. Differential regulation of extracellular matrix protein expression in carcinoma-associated fibroblasts by TGF-beta1 regulates cancer cell spreading but not adhesion. Oncoscience 2014, 1, 634–648.
- Leygue, E.; Snell, L.; Dotzlaw, H.; Troup, S.; Hiller-Hitchcock, T.; Murphy, L.C.; Roughley, P.J.; Watson, P.H. Lumican and decorin are differentially expressed in human breast carcinoma. J. Pathol. 2000, 192, 313–320.
- Leygue, E.; Snell, L.; Dotzlaw, H.; Hole, K.; Hiller-Hitchcock, T.; Roughley, P.J.; Watson, P.H.; Murphy, L.C. Expression of lumican in human breast carcinoma. Cancer Res. 1998, 58, 1348–1352.
- Alowami, S.; Troup, S.; Al-Haddad, S.; Kirkpatrick, I.; Watson, P.H. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003, 5, R129–R135, doi:10.1186/bcr622.
- Kelemen, L.E.; Couch, F.J.; Ahmed, S.; Dunning, A.M.; Pharoah, P.D.; Easton, D.; Fredericksen, Z.; Vierkant, R.A.; Pankratz, V.S.; Goode, E.L.; et al. Genetic variation in stromal proteins decorin and lumican with breast cancer: Investigations in two case-control studies. Breast Cancer Res. 2008, 10, R98, doi:10.1186/bcr2201.
- Ricciardelli, C.; Sakko, A.J.; Ween, M.P.; Russell, D.L.; Horsfall, D.J. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009, 28, 233–245, doi:10.1007/s10555-009-9182-y.
- Kischel, P.; Waltregny, D.; Dumont, B.; Turtoi, A.; Greffe, Y.; Kirsch, S.; De Pauw, E.; Castronovo, V. Versican overexpression in human breast cancer lesions: Known and new isoforms for stromal tumor targeting. Int. J. Cancer 2010, 126, 640–650, doi:10.1002/ijc.24812.
- Canavese, G.; Candelaresi, G.; Castellano, I.; Mano, M. Expression of proteoglycan versican in in situ breast lesions: Relations between stromal changes, histotype, and invasion. Pathol. Res. Pr. 2011, 207, 97–103, doi:10.1016/j.prp.2010.10.009.
- Eshchenko, T.Y.; Rykova, V.I.; Chernakov, A.E.; Sidorov, S.V.; Grigorieva, E.V. Expression of different proteoglycans in human breast tumors. Biochem. Mosc. 2007, 72, 1016–1020, doi:10.1134/s0006297907090143.