Opuntia species are cacti with high ecological, economic and conservation interest in semiarid environments, particularly in Mexico. Despite the economic and cultural importance of Opuntia, there is a significant lack of knowledge about the flower-visiting insects and their taxonomic identity. Although some Opuntia species could be visited by birds such as hummingbirds, the most dominant taxonomic group of pollinators are the insects.
- insecta
- pollination
- drylands
- core species
- conservation
1. Introduction
2. Opuntia (Cactaceae; Opuntioideae) Flower-Visiting Insects
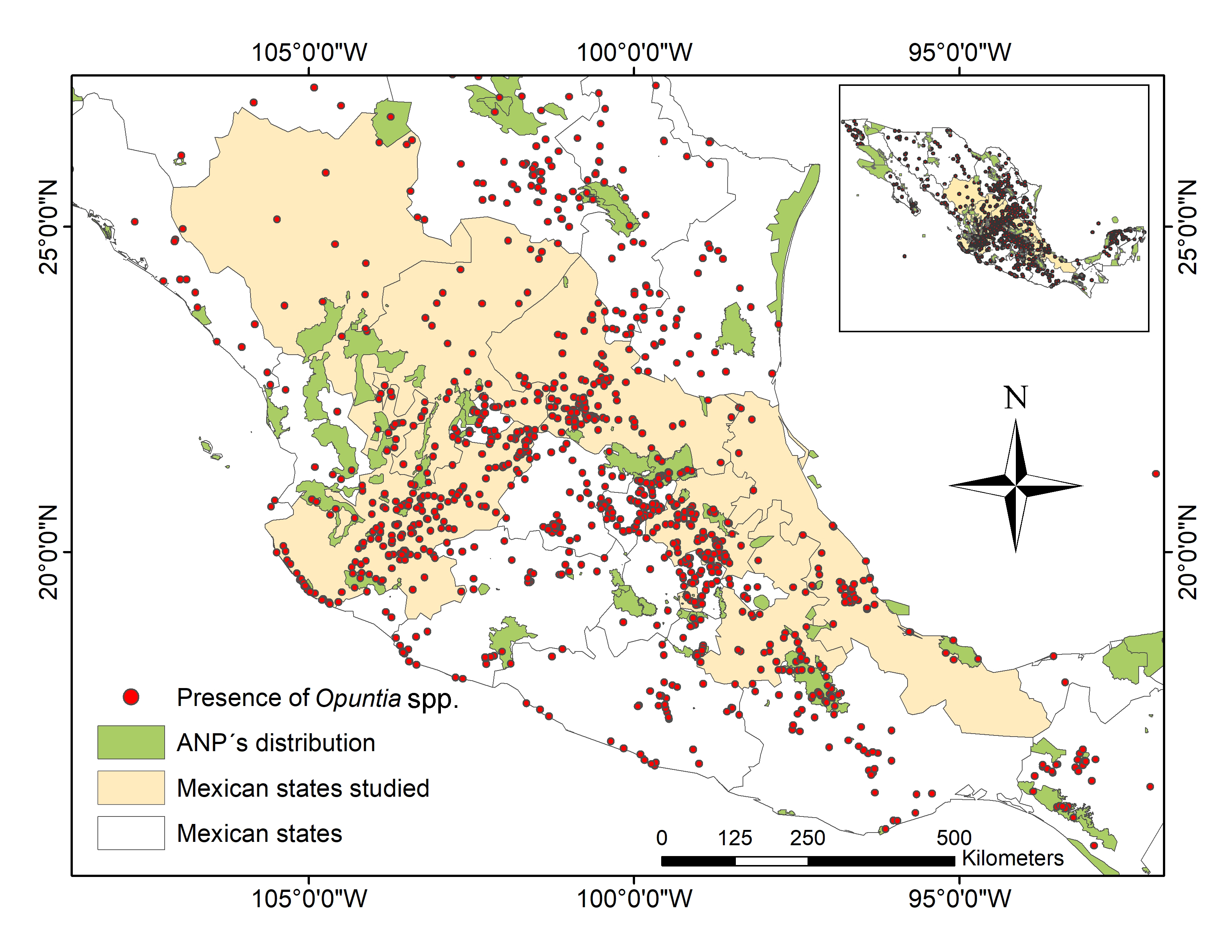
In Mexico, Opuntia species represent one of the most important crops, because they cover about 30% of the country’s land area, and they are mainly distributed throughout arid and semiarid regions (Figure 1) [33]. Mexico is the main producer of prickly pears, supporting 43% of annual world production, which is estimated at 1,060,000 t in an area of 100,000 ha [34]. The area planted with "nopal" in Mexico in 2019 was 45,746 ha, which was mainly in the Mexican High Plateau (central Mexico).
2.1. Timeline of Opuntia Studies
2.2. Insect Diversity: Are All the Species Efficient in Pollen Transport?
2.3. The Relationship between Opuntia and Insects in Mexico
2.4. The Role of Core Species in the Community Structure
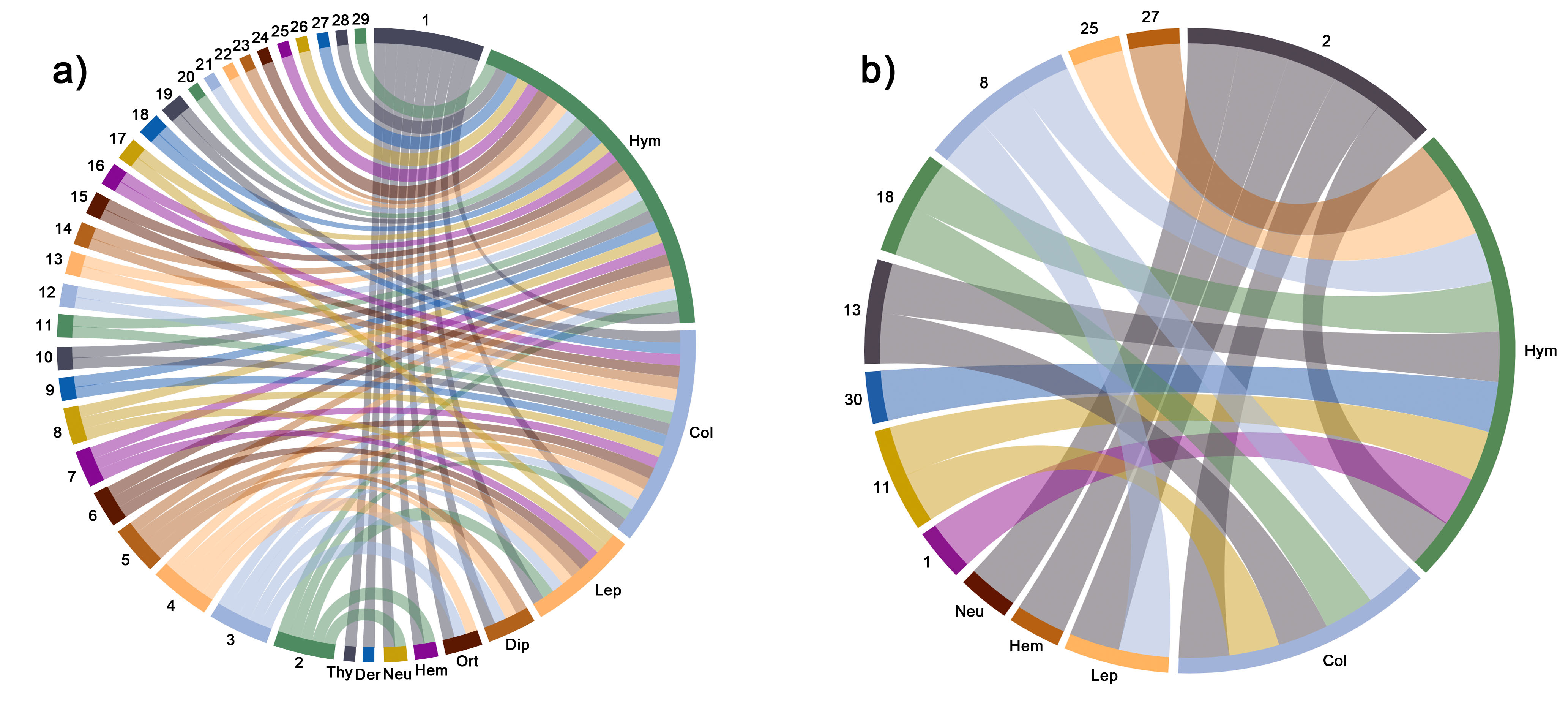
Figure 2. Circular network configuration of flower-visiting orders of insects associated with Opuntia species constructed with the databases of the retrieved articles between 1911 and 2020. Databases were sorted to construct a (a) global network and (b) network of Mexico. Numbers and abbreviations are defined as follows: Hym: Hymenoptera; Col: Coleoptera; Lep: Lepidoptera; Dip: Diptera; Ort: Orthoptera; Hem: Hemiptera; Neu: Neuroptera; Der: Dermaptera; Thy: Thysanoptera. 1: O. ficus-indica; 2: O. pilifera; 3: O: polyacantha; 4: O. fragilis; 5: O. stricta; 6: O. elata; 7: O. maxima; 8: O. tomentosa; 9: O. monacantha; 10: O. lindheimeri; 11: O. robusta; 12: O. anacantha; 13: O. rastrera; 14: O. basilaris; 15: O. humifusa; 16: O. macrorhiza; 17: O. quimilo; 18: O. microdasys; 19: O. littoralis; 20: O. viridirubra; 21: O. spinulifera; 22: O. sulphurea; 23: O. phaeacantha; 24: O. retrorsa; 25: O. streptacantha; 26: O. dillenii; 27: O. huajuapensis; 28: O. macrocentra; 29: O. engelmannii.
3. Conclusions and Future Perspectives
This entry is adapted from the peer-reviewed paper 10.3390/plants11010131
References
- Hernandez-Hernandez, T.; Brown, J.W.; Schlumpberger, B.O.; Eguiarte, L.E.; Magallón, S. Beyond aridification: Multiple explanations for the elevated diversification of cacti in the new world Succulent Biome. New Phytol. 2014, 202, 1382–1397.
- Anderson, E.F. The Cactus Family; Timber Press: Portland, OR, USA, 2001; p. 776.
- Hunt, D. The New Cactus Lexicon; DH Books: Milborne Port, UK, 2006; Volumes 1 and 2, p. 925.
- Guerrero, P.C.; Majure, L.C.; Cornejo-Romero, A.; Hernández-Hernández, T. Phylogenetic relationships and evolutionary trends in the cactus family. J. Hered. 2019, 110, 4–21.
- González-Medrano, F. Las Zonas Áridas y Semiáridas de México y su Vegetación; Instituto Nacional de Ecología–Secretaria del Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2012; p. 194.
- Goettsch, B.; Hilton-Taylor, C.; Cruz-Piñón, G.; Duffy, J.P.; Frances, A.; Hernández, H.M.; Inger, R.; Pollock, C.; Schipper, J.; Superina, M.; et al. High proportion of cactus species threatened with extinction. Nat. Plants 2015, 1, 1–7.
- Jiménez-Sierra, C.L. Las cactáceas mexicanas y los riesgos que enfrentan. Revista Digital Universitaria 2011, 12, 5–13.
- Majure, L.C.; Puente, R. Phylogenetic relationships and morphological evolution in Opuntia s. str. and closely related members of tribe Opuntieae. Succ. Plant Res. 2014, 8, 9–30.
- Majure, L.C.; Puente, R.; Griffith, M.P.; Judd, W.S.; Soltis, P.S.; Soltis, D.E. Phylogeny of Opuntia s.s. (Cactaceae): Clade delineation, geographic origins, and reticulate evolution. Am. J. Bot. 2012, 99, 847–864.
- Aliscioni, N.L.; Delbón, N.E.; Gurvich, D.E. Spine function in Cactaceae, a review. J. Prof. Assoc. Cactus 2021, 23, 1–11.
- González-Elizondo, M.S.; González-Elizondo, M.; López-Enríquez, I.L.; Tena-Flores, J.A.; González-Gallegos, J.G.; Ruacho-González, L.; Melgoza-Castillo, A.; Villarreal-Quintanilla, J.A.; Estrada-Castillón, A.E. Diagnóstico del conocimiento taxonómico y florístico de las plantas vasculares del norte de México. Bot. Sci. 2017, 95, 760–779.
- Flores Valdez, C.A.; Aguirre Rivera, J.R. El Nopal como Forraje; Universidad Autónoma Chapingo: Texcoco, México, 1979; p. 91.
- Anaya-Pérez, M.A.; Bautista-Zane, R. El nopal forrajero en México: Del siglo XVI al siglo XX. Agric. Soc. Desarro. 2008, 5, 167–183.
- Ortega-Baes, P.; Sühring, S.; Sajama, J.; Sotola, E.; Alonso-Pedano, M.; Bravo, S.; Godínez-Alvarez, H. Diversity and conservation in the Cactus family. In Desert Plants. Biology and Biotechnology, 1st ed.; Ramawat, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 157–173.
- Dubeux, J.C.B., Jr.; Dos Santos, M.V.F.; Da Cunha, M.V.; Dos Santos, D.C.; De Almeida Souza, R.T.; De Mello, A.C.L.; De Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed Sci. and Tech. 2021, 275, 114890.
- Ciriminna, R.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Pagliaro, M. Toward unfolding the bioeconomy of nopal (Opuntia spp.). Biofuel Bioprod. Bior. 2019, 13, 1417–1427.
- Le Houerou, H.N. Utilization of fodder trees and shrubs in the arid and semiarid zones of West Asia and North Africa. Arid Soil Res. Rehabil. 2000, 14, 101–135.
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951.
- Bartomeus, I.; Vilà, M. Breeding system and pollen limitation in two supergeneralist alien plants invading Mediterranean shrublands. Aust. J. Bot. 2009, 57, 109–115.
- Padrón, B.; Traveset, A.; Biedenweg, T.; Díaz, D.; Nogales, M.; Olesen, J.M. Impact of alien plant invaders on pollination networks in two archipelagos. PLoS ONE 2009, 4, e6275.
- Instituto Nacional de Estadística y Geografía (INEGI). Características Principales del Cultivo del Nopal en el Distrito Federal Caso Milpa Alta; Censo Agropecuario 2007; Instituto Nacional de Estadística y Geografía: Aguascalientes, Mexico, 2007; p. 68.
- Rebman, J.P.; Pinkava, D.J. Opuntia cacti of North America: An overview. Fla. Entomol. 2001, 84, 474–483.
- Reyes-Agüero, J.A.; Valiente-Banuet, A. Reproductive biology of Opuntia: A review. J. Arid Environ. 2006, 64, 549–585.
- Inglese, P.; Mondragon, C.; Nefzaoui, A.; Saenz, C.; Taguchi, M.; Makkar, H.; Louhaichi, M. Ecologia del Cultivo, Manejo y Usos del Nopal; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018; p. 229. Available online: https://hdl.handle.net/20.500.11766/9380 (accessed on 22 October 2021).
- Mandujano, M.C.; Carrillo-Ángeles, I.; Martínez Peralta, C.; Golubov, J. Reproductive biology of Cactaceae. In Desert Plants. Biology and Biotechnology, 1st ed.; Ramawat, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 197–230.
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112.
- Taki, H.; Kevan, P.G. Does habitat loss affect the communities of plants and insects equally in plant–pollinator interactions? Preliminary findings. Biodivers. Conserv. 2007, 16, 3147–3161.
- Senapathi, D.; Biesmeijer, J.C.; Breeze, T.D.; Kleijn, D.; Potts, S.G.; Carvalheiro, L.G. Pollinator conservation—The difference between managing for pollination services and preserving pollinator diversity. Curr. Opin. Insect Sci. 2015, 12, 93–101.
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353.
- Van der Kooi, C.J.; Vallejo-Marín, M.; Leonhardt, S.D. Mutualisms and (A) symmetry in plant-pollinator interactions. Curr. Biol. 2021, 31, 91–99.
- Morales-Trejo, J.J.; Sandoval-Ruiz, C.A.; Fascinetto-Zago, P.; Cruzado-Lima, A.L.; Vázquez-Hernández, C. Abundancia y diversidad de visitadores florales de Opuntia pilifera en Zapotitlán Salinas, Puebla. Entomol. Mex. 2014, 1, 1144–1148.
- Pimienta, B.E.; Del Castillo, R.F. Reproductive biology. In Cacti: Biology and Uses; Nobel, P.S., Ed.; University of California Press: Los Angeles, CA, USA, 2002; pp. 75–90.
- Gallegos-Vázquez, C.; Méndez-Gallegos, S.D.J.; Mondragón, J.C. Producción Sustentable de Tuna en San Luis Potosí; Colegio de Postgraduados–Fundación Produce San Luis Potosí: San Luis Potosí, Mexico, 2013; p. 203.
- Potgieter, J.; D’Aquino, S. Fruit production and post-harvest management. In Ecologia del Cultivo, Manejo y Usos del Nopal; Inglese, P., Mondragon, C., Nefzaoui, A., Saenz, C., Taguchi, M., Makkar, H., Louhaichi, M., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018; pp. 51–71. Available online: https://hdl.handle.net/20.500.11766/9380 (accessed on 13 December 2021).
- Bembower, W. Pollination notes from the Cedar Point region. Ohio Nat. 1911, 9, 378–383.
- Cockerell, T.D.A. Two new subgenera of north American bees. Am. Mus. Novit 1922, 47, 1–5.
- Grant, V.; Grant, K.A. Pollination of Opunta basilaris and O. littoralis. Plant Syst. Evol. 1979, 132, 321–325.
- Grant, V.; Grant, K.A.; Hurd, P.D. Pollination of Opuntia lindheimeri and related species. Plant Syst. Evol. 1979, 132, 313–320.
- Del Castillo, R.; González-Espinosa, M. Una interpretación evolutiva del polimorfismo sexual de Opuntia robusta (Cactaceae). Agrociencia 1988, 71, 185–196.
- Mandujano, M.D.C.; Montaña, C.; Eguiarte, L.E. Reproductive ecology and inbreeding depression in Opuntia rastrera (Cactaceae) in the Chihuahuan Desert: Why are sexually derived recruitments so rare? Am. J. Bot. 1996, 83, 63–70.
- Fachardo, A.L.S.; Sigrist, M.R. Pre-zygotic reproductive isolation between two synchronopatric Opuntia (Cactaceae) species in the Brazilian Chaco. Plant Biol. 2020, 22, 487–493.
- Pretto, F.; Celesti-Grapow, L.; Carli, E.; Blasi, C. Influence of past land use and current human disturbance on non-native plant species on small Italian islands. Plant Ecol. 2010, 210, 225–239.
- Lo Verde, G.; La Mantia, T. The role of native flower visitors in pollinating Opuntia ficus-indica (L.) Mill., naturalized in Sicily. Acta Oecologica 2011, 37, 413–417.
- Bartomeus, I.; Vilà, M.; Santamaría, L. Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 2008, 155, 761–770.
- Jauker, F.; Speckmann, M.; Wolters, V. Intra-specific body size determines pollination effectiveness. Basic Appl. Ecol. 2016, 17, 714–719.
- Osborn, M.M.; Kevan, P.G.; Lane, M.A. Pollination biology of Opuntia polyacantha and Opuntia phaeacantha (Cactaceae) in southern Colorado. Plant Syst. Evol. 1988, 159, 85–94.
- Cota-Sánchez, J.H.; Almeida, O.J.G.; Falconer, D.J.; Choi, H.J.; Bevan, J. Intriguing thigmonastic (sensitive) stamens in the plains prickly pear Opuntia polyacantha (Cactaceae). Flora 2013, 208, 381–389.
- Schlindwein, C.; Wittmann, D. Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst. Evol. 1997, 204, 179–193.
- Lenzi, M.; Orth, A.I. Floral visitors of the Opuntia monacantha (Cactaceae) in sandbank of the Florianópolis, SC, Brazil. Acta Biológica Paranaense 2011, 40, 19–32.
- Gómez, J.M. Effectiveness of ants as pollinators of Lobularia maritima: Effects on main sequential fitness components of the host plant. Oecologia 2000, 122, 90–97.
- Maubecin, C.C.; Boero, L.; Sérsic, A.N. Specialisation in pollen collection, pollination interactions and phenotypic variation of the oil-collecting bee Chalepogenus cocuccii. Apidologie 2020, 51, 710–723.
- Arroyo-Pérez, E.; Jiménez-Sierra, C.L.; Zavala Hurtado, J.A.; Flores, J. Shared pollinators and sequential flowering phenologies in two sympatric cactus species. Plant Ecol. Evo. 2021, 154, 28–38.
- Samra, S.; Samocha, Y.; Eisikowitch, D.; Vaknin, Y. Can ants equal honeybees as effective pollinators of the energy crop Jatropha curcas L. under Mediterranean conditions? GCB Bioenergy 2014, 6, 756–767.
- Pickett, C.H.; Clark, W.D. The function of extrafloral nectaries in Opuntia acanthocarpa (Cactaceae). Am. J. Bot. 1979, 66, 618–625.
- LeVan, K.E.; Hung, K.L.J.; McCann, K.R.; Ludka, J.T.; Holway, D.A. Floral visitation by the Argentine ant reduces pollinator visitation and seed set in the coast barrel cactus, Ferocactus viridescens. Oecologia 2014, 174, 163–171.
- Mauseth, J.D.; Rebmann, J.P.; Machado, S.R. Extrafloral nectaries in cacti. Cactus Succul. J. 2016, 88, 156–171.
- Wagner, D.; Kay, A. Do extrafloral nectaries distract ants from visiting flowers? An experimental test of an overlooked hypothesis. Evol. Ecol. Res. 2002, 4, 293–305.
- Komamura, R.; Koyama, K.; Yamauchi, T.; Konno, Y.; Gu, L. Pollination contribution differs among insects visiting Cardiocrinum cordatum flowers. Forests 2021, 12, 452.
- Beattie, A.J.; Turnbull, C.; Knox, R.B.; Williams, E.G. Ant inhibition of pollen function—A possible reason why ant pollination is rare. Am. J. Bot. 1984, 71, 421–426.
- Rostás, M.; Bollmann, F.; Saville, D.; Riedel, M. Ants contribute to pollination but not to reproduction in a rare calcareous grassland forb. PeerJ 2018, 6, e4369.
- Rostás, M.; Tautz, J. Ants as pollinators of plants and the role of floral scents. In All Flesh is Grass. Cellular Origin, Life in Extreme Habitats and Astrobiology, 1st ed.; Dubinsky, Z., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 16, pp. 149–161.
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821.
- Ávila-Gómez, E.S.; Meléndez-Ramírez, V.; Castellanos, I.; Zuria, I.; Moreno, C.E. Prickly pear crops as bee diversity reservoirs and the role of bees in Opuntia fruit production. Agric. Ecosyst. Environ. 2019, 279, 80–88.
- Piña, H.H.; Montaña, C.; Mandujano, M. Fruit abortion in the Chihuahuan-Desert endemic cactus Opuntia microdasys. Plant Ecol. 2007, 193, 305–313.
- Santa Anna-Aguayo, A.I.; Schaffner, C.M.; Golubov, J.; López-Portillo, J.; García-Franco, J.; Herrera-Meza, G.; Martínez, A.J. Behavioral repertoires and interactions between Apis mellifera (Hymenoptera: Apidae) and the native bee Lithurgus littoralis (Hymenoptera: Megachilidae) in flowers of Opuntia huajuapensis (Cactaceae) in the Tehuacan desert. Fla. Entomol. 2017, 100, 396–402.
- McCravy, K.W. A review of sampling and monitoring methods for beneficial arthropods in agroecosystems. Insects 2018, 9, 170.
- Montgomery, G.A.; Belitz, M.W.; Guralnick, R.P.; Tingley, M.W. Standards and best practices for monitoring and benchmarking insects. Front. Ecol. Evol. 2021, 8, 579193.
- Vigueras, A.L.; Portillo, L. Uses of Opuntia species and the potential impact of Cactoblastis cactorum (Lepidoptera: Pyralidae) in Mexico. Fla. Entomol. 2001, 84, 493–498.
- Griffith, M.P. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): New molecular evidence. Am. J. Bot. 2004, 91, 1915–1921.
- Barbera, G.; Carimi, F.; Inglese, P. Past and present role of the indian-fig prickly-pear (Opuntia ficus-indica (L) Miller, Cactaceae) in the agriculture of Sicily. Econ. Bot. 1992, 46, 10–20.
- Stang, M.; Klinkhamer, P.G.; Van Der Meijden, E. Size constraints and flower abundance determine the number of interactions in a plant–flower visitor web. Oikos 2006, 112, 111–121.
- Lopezaraiza-Mikel, M.E.; Hayes, R.B.; Whalley, M.R.; Memmott, J. The impact of an alien plant on a native plant–pollinator network: An experimental approach. Ecol. Lett. 2007, 10, 539–550.
- Ness, J.H. Hot spots and hot moments for on-plant foraging by ants within the flora of warm North American Deserts. Am. Midl. Nat. 2020, 183, 145–163.
- Vithanage, V. The role of the European honeybee (Apis mellifera L.) in avocado pollination. J. Hortic. Sci. 1990, 65, 81–86.
- Rizzardo, R.A.; Milfont, M.O.; Silva, E.; Freitas, B.M. Apis mellifera pollination improves agronomic productivity of anemophilous castor bean (Ricinus communis). Anais da Academia Brasileira de Ciências 2012, 84, 1137–1145.
- Moritz, R.F.; Härtel, S.; Neumann, P. Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Ecoscience 2005, 12, 289–301.
- De la Rúa, P.; Paxton, R.J.; Moritz, R.F.A.; Roberts, S.; Allen, D.J.; Pinto, M.A.; Cauia, E.; Fontana, P.; Kryger, P.; Bouga, M.; et al. Apis mellifera. The IUCN Red List of Threatened Species 2014, e.T42463639A42463665. Available online: https://ec.europa.eu/environment/nature/conservation/species/redlist/downloads/European_bees.pdf (accessed on 13 March 2021).
- Paudel, Y.P.; Mackereth, R.; Hanley, R.; Qin, W. Honeybees (Apis mellifera L.) and pollination issues: Current status, impacts, and potential drivers of decline. J. Agric. Sci. 2015, 7, 93–109.
- Grixti, J.C.; Wong, L.T.; Cameron, S.A.; Favret, C. Decline of bumble bees (Bombus) in the North American Midwest. Biol. Conserv. 2009, 142, 75–84.
- Jacobson, M.M.; Tucker, E.M.; Mathiasson, M.E.; Rehan, S.M. Decline of bumble bees in northeastern North America, with special focus on Bombus terricola. Biol. Conserv. 2018, 217, 437–445.
- Hatfield, R.; Jepsen, S.; Thorp, R.; Richardson, L.; Colla, S.; Foltz Jordan, S. Bombus pensylvanicus. The IUCN Red List of Threatened Species 2015, e.T21215172A21215281. Available online: https://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T21215172A21215281.en (accessed on 25 February 2021).
